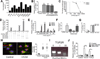TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement - PubMed (original) (raw)
. 2013 Dec;16(12):1773-82.
doi: 10.1038/nn.3560. Epub 2013 Oct 27.
Affiliations
- PMID: 24162655
- PMCID: PMC3973738
- DOI: 10.1038/nn.3560
TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement
Allison R Bialas et al. Nat Neurosci. 2013 Dec.
Retraction in
- Retraction Note: TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement.
Bialas AR, Stevens B. Bialas AR, et al. Nat Neurosci. 2022 Feb;25(2):265. doi: 10.1038/s41593-021-00877-7. Nat Neurosci. 2022. PMID: 35027764 Free PMC article. No abstract available.
Abstract
Immune molecules, including complement proteins C1q and C3, have emerged as critical mediators of synaptic refinement and plasticity. Complement localizes to synapses and refines the developing visual system through C3-dependent microglial phagocytosis of synapses. Retinal ganglion cells (RGCs) express C1q, the initiating protein of the classical complement cascade, during retinogeniculate refinement; however, the signals controlling C1q expression and function remain elusive. Previous work implicated an astrocyte-derived factor in regulating neuronal C1q expression. Here we identify retinal transforming growth factor (TGF)-β as a key regulator of neuronal C1q expression and synaptic pruning in the developing visual system. Mice lacking TGF-β receptor II (TGFβRII) in retinal neurons had reduced C1q expression in RGCs and reduced synaptic localization of complement, and phenocopied refinement defects observed in complement-deficient mice, including reduced eye-specific segregation and microglial engulfment of RGC inputs. These data implicate TGF-β in regulating neuronal C1q expression to initiate complement- and microglia-mediated synaptic pruning.
Figures
Figure 1. C1q is rapidly upregulated in neurons in response to astrocyte-secreted factors
(A) QPCR results for c1qb showed increased c1qb expression relative to controls (RGCs treated with control media) after 6-day treatment with astrocyte insert or astrocyte conditioned medium (ACM). ACM prepared from purified cortical astrocytes (cortical), purified retinal astrocytes (retinal), or astrocytes prepared using the McCarthy and Devellis protocol (MD) all showed a similar upregulation (one-way ANOVA, n=3 experiments, p<0.0001, F(5,12)=75.41). (B) C1q upregulation timecourse for all three C1q genes (c1qa, c1qb, and c1qc) (two-way ANOVA, n=4 experiments,***p<0.001, *p<0.05, F(4,75)=34.37). (C) QPCR results for c1qb showed increased c1qb expression relative to control in RGC cultures but not in microglia or astrocyte cultures after treatment with ACM for 15 min. (two way ANOVA, n=3 experiments, p<0.005, F(2,18)=5.71). (D) Western blot showed an increase in C1q protein in RGC media after 6 days of treatment with ACM. Results shown are representative of 3 experiments. Full length blots are displayed in Supplemental Figure 6. (E) There is a corresponding increase in C1q protein within 6 hours of adding ACM to cultures detected by immunohistochemistry (rabbit anti-mouse C1qA). Scale bar = 100um. (F) Quantification showed increased C1q levels in ACM treated cultures measured as a change in fluorescence intensity relative to control untreated cultures (t test, n=3 experiments, p<0.01, t(4)=37.48).
Figure 2. TGF-β is necessary and sufficient for neuronal C1q upregulation in vitro
(A) ACM cytokine profiling by ELISA. N=3 independent ACM batches. (B) Immunodepletion of TGF-β, but not other cytokines, significantly reduced ACM-induced C1q upregulation (one-way ANOVA, n= 3 experiments, ***p<0.001, **p<0.01, F(4,12)=22.15). (C) Validation of immunodepletion. %Neutralization represents ([TGF-β]initial – [TGF-β]depletion)/(initial TGF-β concentration). N=3 experiments. (D) Immunodepletion of each TGF-β isoform showed that depletion of pan-TGF-β or TGF-β3 blocked C1q upregulation (two-way ANOVA, n=3 experiments, ***p<0.001, F(4,20)=79.52). (E) Concentration-response curves for TGF-β1, 2, and 3 (Two-way ANOVA, n= 3 experiments, ****p<0.0001, **p<0.01, F(10,36)=23.56). (F) Glycine elution showed that either anti-pan TGF-β or anti-TGF-β3 eluates upregulate C1q (one-way ANOVA, n= 3 experiments, ***p<0.001, F(4,10)=11.02). (G) QPCR results for c1qb in RGC cultures and in microglia or retinal astrocyte cultures after TGF-β3 treatment (50 pg/ml) for 15 min. (two way ANOVA, n=3 experiments, p<0.05, F(2,12)=415.96). (H) RGCs showed increased nuclear accumulation of pSmad2 (15–30 min. ACM treatment). Quantification showed a significant increase in pSmad (red) within the nuclear area (blue) (one-way ANOVA, n= 15 cells/condition, ***p<0.001, F(2,9)=19.83). Scale bar = 20um. (I) RT-PCR for tgfbr1, tgfbr2, and tgfbr3 in P5 retina. Data shown are representative of 4 samples tested. Full length gels are displayed in Supplemental Fig. 6. (J) Blocking TGFβRII signaling with neutralizing antibodies or with inhibitors of TGFβRI significantly reduced the effects of ACM or TGF-β3 (0.05 ng/ml) on C1q (two-way ANOVA, n= 3 experiments, **p<0.01, ***p<0.001, F(4,18)=35.06).
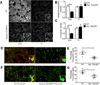
Figure 3. TGF-β expression corresponds to synaptic refinement period in the retinogeniculate system
(A) RT-PCR showed expression of all three TGF-β isoforms in the P5 mouse retina. Data are representative of 4 mice. (B) Western blot for TGFβRII (goat anti-human TGFβRII, R&D systems) showed developmental expression of TGFβRII in the mouse retina. See Supplemental Fig. 6 for full length blot. (C) Relative intensity quantification normalized to beta-actin control for each age showed developmental TGFβRII expression in the postnatal mouse retina (one-way ANOVA, n=3 experiments, **p<0.01, F(2,6)=26.36). (D) Immunostaining with antibodies against TGFβRII (R&D systems, goat anti-TGFβRII) showed that the receptor localizes to the RGC layer and the IPL (arrows) and that staining intensity is dramatically reduced at P15 relative to P5. Antibody staining was confirmed for specificity by staining retinal _TGFβRII_−/− mice. All images were obtained with set exposure times. Scale bar = 50um. (E) RT-PCR confirmed the absence of tgfbr2 mRNA in RGCs acutely isolated from P5 mice using immunopanning. Data shown are representative of 4 animals tested. Full length gel in Supplemental Fig. 6. (F) Immunohistochemistry for total Smad2 showed no difference in relative fluorescence intensity (RGC layer) in WT littermates and retinal _TGFβRII_−/− mice (t test, n=3 mice/genotype, p=0.96 (ns), t(4)=0.053). Scale bar = 50µm. (G) Immunohistochemistry for phosphorylated Smad (pSmad). Co-staining for an RGC marker, Brn3a, and pSmad2/3 showed a significant reduction in pSmad levels quantified in RGCs specifically (t test, n= 3 mice/group, p<0.001, t(4)=13.18). Scale bar = 50µm.
Figure 4. TGF-β signaling is required for neuronal C1q expression in vivo
(A) In situ hybridization for c1qb showed significantly reduced C1q expression in the RGC layer (arrows) in retinal _TGFβRII_−/− mice. Scale bar = 100µm. (B) RGCs acutely isolated from P5 WT (white bar) and retinal _TGFβRII_−/− (grey bar) retinas using immunopanning showed significantly reduced C1q expression. Microglia acutely isolated using CD45 immunopanning showed no significant difference in C1q levels (two-way ANOVA, n= 5 mice/group, **p<0.01, F(1,16)=19.11). (C) Immunostaining for C1q in P5 retina. (Scale bar = 50µm). In retinal _TGFβRII_−/− mice, C1q localization to the RGC layer (inset a vs. d), the IPL (b vs. e) is reduced relative to WT animals. Retinal microglia showed no change in C1q levels (inset c and f). (D) Quantification of the relative fluorescence intensity in each retinal area in WT littermates and retinal _TGFβRII_−/− mice showed significantly reduced C1q localization to the RGC layer and IPL when TGF-β signaling was blocked (two-way ANOVA, n= 4 mice/group, *p<0.05, F(2,12)=11.69). (E) Immunohistochemistry for C1q in optic nerve cross sections showed C1q-immunopositive puncta co-localized with RGC axon fascicles labeled by TUJ1 (Green). C1q levels were significantly reduced in the retinal _TGFβRII_−/− mouse within the axon, as indicated by co-localization with TUJ1. C1q labeling of microglia remained unchanged (arrows). Scale bar = 10µm. (F) Quantification of the fluorescence intensity for C1q staining with axon bundles shows a significant decrease in C1q levels (t test, n=3 mice (two nerves per mouse), p=0.0055, t(6)=4.225).
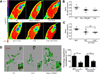
Figure 5. Retinal TGF-β signaling is required for complement localization in the dLGN
(A) C1q immunohistochemistry in the dLGN and primary visual cortex (V1) shows reduced C1q fluorescence intensity in the dLGN but not in V1. Scale bar = 20µm. Inset shows 3× magnification. (B) Quantifying relative fluorescence intensity showed significant reduced intensity in the dLGN of retinal _TGFβRII_−/− vs. WT mice (two-way ANOVA, n=4 mice/group, **p<0.01, F(1,12)=11.21). No differences were observed in V1. (C) C1q puncta density quantification showed significantly reduced C1q puncta density in the dLGN in retinal _TGFβRII_−/− mice vs. WT littermates (two-way ANOVA, n=4 d C1q expression. Microglia acutely isolated using CD45 immunopanning showed no significant difference in C1q levels (two-way ANOVA, n= 5 mice/group, *p<0.05, F(1,8)=7.48). No difference in C1q puncta density was observed in V1. (D) C1q localization to vglut2-positive RGC terminals is reduced in the retinal _TGFβRII_−/− mouse. Immunostaining for C1q and vglut2 in WT and retinal _TGFβRII_−/− P5 dLGNs showed a reduction in co-localization of C1q and vglut2. Scale bar = 20µm. Star indicates the enlarged synaptic puncta shown in the inset. (E) Quantification of C1q co-localization with vglut2 showed significantly reduced synaptic localization of C1q in retinal _TGFβRII_−/− mice. Co-localized puncta were identified and counted using ImageJ Puncta Analyzer (t test, n=4 mice, *p=0.0226, t(6)=3.045). (F) C3 localization to vglut2-positive RGC terminals is reduced in the retinal _TGFβRII_−/− mice, similar to what we observed for C1q. Scale bar = 20µm. (G) Co-localized C3 and vglut2 puncta were identified and counted using ImageJ Puncta Analyzer (t test, n=4 mice, *p<0.0395, t(6)=2.622).
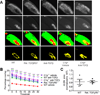
Figure 6. TGF-β signaling and C1q are required for eye specific segregation and microglia-mediated pruning in the retinogeniculate system
(A) Representative dLGN images for WT, retinal _TGFβRII_−/−, _C1q_−/−, IgG1 control, and anti-TGF-β injected WT and _C1q_−/− mice pseudocolored to show the _R_-value for each pixel. _R_=log10(_F_Ipsi/_F_Contra). Scale bar = 100µm. (B) Quantification of the mean variance of the R-value for each group. A significant reduction in the mean variance of the R value is seen in mice deficient in TGF-β signaling or C1q (one-way ANOVA, n= 6 animals/group, **p<0.01, F(2,15)=8.228). (C) There is no additional decrease in the R-value variance when TGF-β signaling is blocked in _C1q_−/− mice. Data shown as mean R-value variance +/− SEM (one-way ANOVA, n= 6 animals/group, *p<0.05, F(2,15)=8.567). (D) Microglia show reduced engulfment of RGC terminals in mice deficient in C1q or retinal TGF-β signaling. Volume of each microglia and the engulfed CTB was quantified in Imaris and the % engulfment defined as the volume of internalized CTB/volume of microglia. Results were normalized to WT engulfment levels and _C1q_−/− or retinal TGFβRII−/− mice both showed a significant reduction in % engulfment (one-way ANOVA, n= 6 mice/group, **p<0.01, F(3,20)=13.66). Scale bar = 10µm. Insets show enlargement of boxed area.
Figure 7. Mice deficient in C1q or retinal TGF-β signaling show increased overlap of contralateral and ipsilateral areas in the dLGN
(A) Representative images of anterograde tracing (Alexa conjugated b-cholera toxin) of contralateral (top row) and ipsilateral (second row) retinogeniculate projections, merged channels (third row) and their overlap (yellow, bottom row) in the dLGN for WT, retinal _TGFβRII_−/−, _C1q_−/−, and anti-TGF-β injected WT and _C1q_−/− mice. Scale bar = 100µm. (B) Quantification of percentage of dLGN area receiving input from both contralateral and ipsilateral eyes (yellow area). Data shown as mean yellow area +/− SEM (two-way ANOVA, n= 6 animals/group, ***p<0.001, **p<0.01, *p<0.05, F(4,175)=101.00). (C) Measurements of dLGN area in P10 mice showed no significant difference between WT and retinal _TGFβRII_−/− mice. Results were normalized to WT dLGN area and 4 dLGNs were analyzed per mouse (t test, n=4 mice, p=0.9342(ns), t(6)=0.08611).
Comment in
- From neurons to microglia, with complements.
Derecki NC, Kipnis J. Derecki NC, et al. Nat Neurosci. 2013 Dec;16(12):1712-3. doi: 10.1038/nn.3579. Nat Neurosci. 2013. PMID: 24270269 Retracted. No abstract available.
Similar articles
- From neurons to microglia, with complements.
Derecki NC, Kipnis J. Derecki NC, et al. Nat Neurosci. 2013 Dec;16(12):1712-3. doi: 10.1038/nn.3579. Nat Neurosci. 2013. PMID: 24270269 Retracted. No abstract available. - The classical complement cascade mediates CNS synapse elimination.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. Stevens B, et al. Cell. 2007 Dec 14;131(6):1164-78. doi: 10.1016/j.cell.2007.10.036. Cell. 2007. PMID: 18083105 - Complement C1q/C3-CR3 signaling pathway mediates abnormal microglial phagocytosis of synapses in a mouse model of depression.
Han QQ, Shen SY, Liang LF, Chen XR, Yu J. Han QQ, et al. Brain Behav Immun. 2024 Jul;119:454-464. doi: 10.1016/j.bbi.2024.04.018. Epub 2024 Apr 18. Brain Behav Immun. 2024. PMID: 38642614 - C1q: the perfect complement for a synaptic feast?
Perry VH, O'Connor V. Perry VH, et al. Nat Rev Neurosci. 2008 Nov;9(11):807-11. doi: 10.1038/nrn2394. Epub 2008 Oct 1. Nat Rev Neurosci. 2008. PMID: 18827829 Review. - The Role of Complement in Synaptic Pruning and Neurodegeneration.
Gomez-Arboledas A, Acharya MM, Tenner AJ. Gomez-Arboledas A, et al. Immunotargets Ther. 2021 Sep 24;10:373-386. doi: 10.2147/ITT.S305420. eCollection 2021. Immunotargets Ther. 2021. PMID: 34595138 Free PMC article. Review.
Cited by
- Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma.
Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, John SW, Howell GR. Williams PA, et al. Mol Neurodegener. 2016 Apr 6;11:26. doi: 10.1186/s13024-016-0091-6. Mol Neurodegener. 2016. PMID: 27048300 Free PMC article. - When glia meet induced pluripotent stem cells (iPSCs).
Li L, Shi Y. Li L, et al. Mol Cell Neurosci. 2020 Dec;109:103565. doi: 10.1016/j.mcn.2020.103565. Epub 2020 Oct 14. Mol Cell Neurosci. 2020. PMID: 33068719 Free PMC article. Review. - EF1α-associated protein complexes affect dendritic spine plasticity by regulating microglial phagocytosis in Fmr1 knock-out mice.
Su P, Yan S, Chen K, Huang L, Wang L, Lee FHF, Zhou H, Lai TKY, Jiang A, Samsom J, Wong AHC, Yang G, Liu F. Su P, et al. Mol Psychiatry. 2024 Apr;29(4):1099-1113. doi: 10.1038/s41380-023-02396-2. Epub 2024 Jan 11. Mol Psychiatry. 2024. PMID: 38212373 - Microglia-leucocyte axis in cerebral ischaemia and inflammation in the developing brain.
Rayasam A, Fukuzaki Y, Vexler ZS. Rayasam A, et al. Acta Physiol (Oxf). 2021 Sep;233(1):e13674. doi: 10.1111/apha.13674. Epub 2021 May 30. Acta Physiol (Oxf). 2021. PMID: 33991400 Free PMC article. Review. - Astrocytes in schizophrenia.
Notter T. Notter T. Brain Neurosci Adv. 2021 Apr 27;5:23982128211009148. doi: 10.1177/23982128211009148. eCollection 2021 Jan-Dec. Brain Neurosci Adv. 2021. PMID: 33997293 Free PMC article. Review.
References
- Stevens B, et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell. 2007;131:1164–1178. - PubMed
- Sretavan D, Shatz CJ. Prenatal development of individual retinogeniculate axons during the period of segregation. Nature. 1984;308:845–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DA-15043/DA/NIDA NIH HHS/United States
- R01 DA015043/DA/NIDA NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- P30-HD-18655/HD/NICHD NIH HHS/United States
- R01-NS-07100801/NS/NINDS NIH HHS/United States
- R01 NS071008/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous