The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence - PubMed (original) (raw)
Review
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence
Jeffrey M Witkin et al. Pharmacol Ther. 2014 Mar.
Erratum in
- Pharmacol Ther. 2014 Sep;143(3):351
Abstract
Nociceptin/Orphanin FQ (N/OFQ) is a 17 amino acid peptide that was deorphanized in 1995. The generation of specific agonists, antagonists and receptor deficient mice and rats has enabled progress in elucidating the biological functions of N/OFQ. Additionally, radio-imaging technologies have been advanced for investigation of this system in animals and humans. Together with traditional neurobehavioral techniques, these tools have been utilized to identify the biological significance of the N/OFQ system and its interacting partners. The present review focuses on the role of N/OFQ in the regulation of feeding, body weight homeostasis, stress, the stress-related psychiatric disorders of depression and anxiety, and in drug and alcohol dependence. Critical evaluation of the current scientific preclinical literature suggests that small molecule modulators of nociceptin opioid peptide receptors (NOP) might be useful in the treatment of diseases related to these biological functions. In particular, the literature data suggest that antagonism of NOP receptors will produce anti-obesity and antidepressant activities in humans. However, there are also contradictory data discussed. The current literature on the role of N/OFQ in anxiety and addiction, on the other hand points primarily to a role of agonist modulation being potentially therapeutic. Some drug-like molecules that function either as agonists or antagonists of NOP receptors have been optimized for human clinical study to test some of these hypotheses. The discovery of PET ligands for NOP receptors, combined with the pharmacological tools and burgeoning preclinical data set discussed here bodes well for a rapid advancement of clinical understanding and potential therapeutic benefit.
Keywords: (1S,3aS)-8- (2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one, a NOP receptor agonist; (±)trans-1-[1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one, a NOP receptor antagonist; 2-{3-[1-((1R)-acenaphthen-1-yl)piperidin-4-yl]-2,3-dihydro-2-oxo-benzimidazol-1-yl}-N-methylacetamide, a NOP receptor agonist; 5-HT; 5-hydroxytryptamine or serotonin; 8-[bis(2-methylphenyl)-methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol; ACTH; Alcohol-preferring rats; Anxiety; BED; BNST; CGRP; CPP; CRF; CTA; Calcitonin gene related peptide; CeA; DA; Depression; Drug dependence; EPSC; FST; G-protein activated, inwardly rectifying K(+) channel; G-protein-coupled receptor; GIRK; GPCR; HPA; J-113397; JTC-801; KO; MDD; Marchigian Sardinian Alcohol-Preferring; N-(4-amino-2-methylquinolin-6-yl)-2-(4-ethylphenoxymethyl)benzamide hydrochloride, a NOP receptor antagonist; N/OFQ; NAcc; NE; NOP; NPY; Nociceptin opioid peptide or Nociceptin opioid peptide receptor; Nociceptin/Orphanin FQ; Nociceptin/Orphanin FQ (F: phenylalanine, Q: glutamine, the amino acids that begin and end the peptide sequence); ORL; Obesity; P rats; POMC; Pro-opiomelanocortin; Ro 64-6198; SB-612111; SCH 221510; SCH 655842; Stress; TST; UFP-101; VTA; W212393; [(–)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol, a NOP receptor antagonist; [Nphe(1),Arg(14),Lys(15)]N/OFQ-NH(2), a NOP receptor antagonist; adrenocorticotropic hormone; bed nucleus of stria terminalis; binge eating disorder; central nucleus of the amygdala; conditioned place preference; conditioned taste aversion; corticotrophin-releasing factor; dopamine; endo-8-[bis(2-chlorophenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octane-3-carboxamide, a NOP receptor agonist; excitatory post-synaptic current; forced-swim test; hypothalamic–pituitary axis; knockout; mPFC; major depressive disorder; medial prefrontal cortex; msP; neuropeptide Y; norepinephrine; nucleus accumbens; opioid-receptor-like; tail-suspension test; ventral tegmental area.
© 2013.
Figures
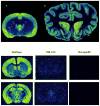
Figure 1
Top: Autoradiographic binding of in striatal sections (12 μM) of (A) rat and (B) dog brain. Sections were incubated with [3H]nociceptin (200 pM) for 120 min at room temperature in buffer containing 50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, and 0.1% bovine serum albumin. Sections were washed 2X for 10 min in ice cold incubation buffer containing no bovine serum albumin or radioligand, followed by a rinse in ice cold distilled water. Sections were exposed for 3 days at room temperature. Color intensity denotes the degree of NOP receptor binding sites with red/orange and yellow colors representing areas of high receptor density and blue representing areas of low receptor density. Note the difference in [3H]nociceptin binding site density within the caudate nucleus (a) exemplifying profound species-related differences in NOP receptor expression. Bottom: Autoradiographic binding of the NOP antagonist tracer [3H]NOP-1A (0.6 nM) in 12 μm coronal sections of wildtype 129/S6 and ORL1 knockout mouse brain according to Pike et al. (2011). Nonspecific binding was determined in the presence of 10 μM SB612111 (from an adjacent section of wildtype mouse). Wildtype 129/S6 mice exhibited high binding in numerous cortical regions (A), medial septum (B), dorsal endopiriform nucleus (C) hippocampus (D), vetromedial hypothalamic nuclues (E), amygdala (F), and central thalamus (G). No appreciable specific binding was observed in equivalent sections of ORL1 knockout mouse brain.
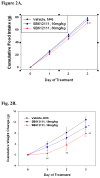
Figure 2
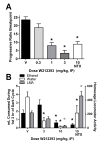
The NOP receptor antagonist SB612111 produces a dose-dependent inhibition of (A) food intake and (B) body weight gain in lean Long-Evans rats (n = 6 rats/group) eating a high fat/high sugar diet (Teklad Research Diets, TD95217). Following initial presention of the TD95217 diet animals become hyperphagic and rapidly gain weight. Animals were treated once daily with SB612111 by the oral route for 3 consecutive days and daily food intake and body weight were measured. * p<0.01; ** p<0.001 vs vehicle treated rats. (C) The NOP receptor antagonist SB612111 in ORL1 knockout mice inhibits fasting-induced 1 hr food intake in a genotype-dependent manner. ORL1 knockout mice and wildtype 129/S6 littermates (n = 6–8 mice/group) were fasted overnight and presented chow 1 hour into the light cycle. The NOP antagonist SB612111 was administered by oral route 1 hr prior to the presentation of food. The amount of food consumed was measured for 1 hr following food presentation, and represents the period during which the most rapid food consumption occurs. * p<0.05; # p<0.05 vs vehicle treated wild type mice.
Figure 2
The NOP receptor antagonist SB612111 produces a dose-dependent inhibition of (A) food intake and (B) body weight gain in lean Long-Evans rats (n = 6 rats/group) eating a high fat/high sugar diet (Teklad Research Diets, TD95217). Following initial presention of the TD95217 diet animals become hyperphagic and rapidly gain weight. Animals were treated once daily with SB612111 by the oral route for 3 consecutive days and daily food intake and body weight were measured. * p<0.01; ** p<0.001 vs vehicle treated rats. (C) The NOP receptor antagonist SB612111 in ORL1 knockout mice inhibits fasting-induced 1 hr food intake in a genotype-dependent manner. ORL1 knockout mice and wildtype 129/S6 littermates (n = 6–8 mice/group) were fasted overnight and presented chow 1 hour into the light cycle. The NOP antagonist SB612111 was administered by oral route 1 hr prior to the presentation of food. The amount of food consumed was measured for 1 hr following food presentation, and represents the period during which the most rapid food consumption occurs. * p<0.05; # p<0.05 vs vehicle treated wild type mice.
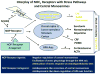
Figure 3
Interplay of NOP receptors with stress pathways, and neuropeptide and non-peptide neurotransmitters. Graphics are drawn from data in the scientific literature cited in the text.
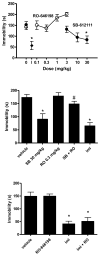
Figure 4
Anti-immobility effects of the NOP receptor antagonist SB-61211 in the mous forced-swim assay. TOP. Lack of effect of NOP receptor agonist RO-646198. NIH Swiss, male mice, n=4–6/group. *p<0.05 compared to vehicle (Dunnett’s test). I: Effect of 15 mg/kg imipramine. MIDDLE. Prevention of effects of SB-61211(30 mg/kg, p.o.) by RO-646198 (0.3 mg/kg). NIH Swiss male mice, n=8/group. *p<0.05 compared to vehicle (Dunnett’s test); #p<0.05 compared to 2817412 alone (t-test). Imi: imipramine (15mg/kg, i.p., n=4), 30 min prior). LOWER. RO-646198 (0.3 mg/kg, p.o.) was not effective in preventing the anti-immobility effects of imipramine (15 mg/kg, i.p.). NIH Swiss male mice, n=8/group. *p<0.05 compared to vehicle (Dunnett’s test). Behavioral methods are comparable to Li et al. (2005).
Figure 5
Effects of the NOP receptor agonist W212393 on ethanol self-administration in Alcohol-Preferring (P) rats. Panel A: data from P rats maintained under a progressive ratio operant schedule of reinforcement (0.1 ml 15% ethanol v/v in water/reinforcer). Panel B: data in P rats with continuous homecage access to ethanol (15% v/v in water) and water. Methods used for homecage ethanol drinking in P rats were similar to those reported in Rorick-Kehn et al. (Neuropharmacology, in press), except that W212393 was dissolved in 20% Captisol in phosphate buffer and dosed IP immediately before onset of the dark cycle, using a within-subjects design (exception: W212393 was dosed 30 min prior to progressive ratio operant experiments). Homecage locomotor activity is shown on the right y-axis (gray bars). *p<0.05 compared to vehicle (V). NTX = naltrexone, LMA = locomotor activity.
Figure 6
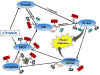
Circuitry underlying ethanol addiction as controlled by NOP receptors. The diagram represents neuroanatomical, neurochemical, and electrophysiological evidence describing modulation of mesocortical and mesolimbic dopamine circuits by NOP receptors, and their relationship to KOP and MOP receptors. Blue ovals = nuclei; ↑ = excitatory connection; T = inhibitory connection. Abbreviations: NOP, nociceptin opioid receptor; MOP, mu opioid receptor; KOP, kappa opioid receptor; VTA, ventral tegmental area, NAcc, nucleus accumbens; BNST, bed nucleus of the stria terminalis; GABA, g-aminobutyric acid; CRF = corticotropin releasing factor.
Similar articles
- Ro 64-6198 [(1S,3aS)-8-(2,3,3a,4,5,6-Hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one] acts differently from nociceptin/orphanin FQ in rat periaqueductal gray slices.
Chiou LC, Chuang KC, Wichmann J, Adam G. Chiou LC, et al. J Pharmacol Exp Ther. 2004 Nov;311(2):645-51. doi: 10.1124/jpet.104.070219. Epub 2004 Jul 13. J Pharmacol Exp Ther. 2004. PMID: 15254141 - Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs.
Gavioli EC, Calo' G. Gavioli EC, et al. Pharmacol Ther. 2013 Oct;140(1):10-25. doi: 10.1016/j.pharmthera.2013.05.008. Epub 2013 May 24. Pharmacol Ther. 2013. PMID: 23711793 Review. - Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 [(-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol]: in vitro studies.
Spagnolo B, Carrà G, Fantin M, Fischetti C, Hebbes C, McDonald J, Barnes TA, Rizzi A, Trapella C, Fanton G, Morari M, Lambert DG, Regoli D, Calò G. Spagnolo B, et al. J Pharmacol Exp Ther. 2007 Jun;321(3):961-7. doi: 10.1124/jpet.106.116764. Epub 2007 Feb 28. J Pharmacol Exp Ther. 2007. PMID: 17329552 - Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder.
Zhang Y, Simpson-Durand CD, Standifer KM. Zhang Y, et al. Br J Pharmacol. 2015 Jan;172(2):571-82. doi: 10.1111/bph.12701. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24666365 Free PMC article. - Nociceptin/orphanin FQ peptide receptors: pharmacology and clinical implications.
Chiou LC, Liao YY, Fan PC, Kuo PH, Wang CH, Riemer C, Prinssen EP. Chiou LC, et al. Curr Drug Targets. 2007 Jan;8(1):117-35. doi: 10.2174/138945007779315605. Curr Drug Targets. 2007. PMID: 17266536 Review.
Cited by
- Parsing the hedonic and motivational influences of nociceptin on feeding using licking microstructure analysis in mice.
Mendez IA, Maidment NT, Murphy NP. Mendez IA, et al. Behav Pharmacol. 2016 Sep;27(6):516-27. doi: 10.1097/FBP.0000000000000240. Behav Pharmacol. 2016. PMID: 27100061 Free PMC article. - Opioid modulation of cognitive impairment in depression.
Jacobson ML, Wulf HA, Browne CA, Lucki I. Jacobson ML, et al. Prog Brain Res. 2018;239:1-48. doi: 10.1016/bs.pbr.2018.07.007. Epub 2018 Sep 18. Prog Brain Res. 2018. PMID: 30314565 Free PMC article. Review. - Methanol extract of semen Ziziphi Spinosae attenuates ethanol withdrawal anxiety by improving neuropeptide signaling in the central amygdala.
Li LB, Kim YW, Wang YH, Bai L, Zhu XD, Zhao ZL, Lee CW, Jiao Y, Wu T, Cai ZZ, Kim SC, An WG, Yang CH, Cui GC, Zhao RJ. Li LB, et al. BMC Complement Altern Med. 2019 Jun 24;19(1):147. doi: 10.1186/s12906-019-2546-0. BMC Complement Altern Med. 2019. PMID: 31234859 Free PMC article. - NOP-Related Mechanisms in Substance Use Disorders.
Ciccocioppo R, Borruto AM, Domi A, Teshima K, Cannella N, Weiss F. Ciccocioppo R, et al. Handb Exp Pharmacol. 2019;254:187-212. doi: 10.1007/164_2019_209. Handb Exp Pharmacol. 2019. PMID: 30968214 Free PMC article. - Deficient endocannabinoid signaling in the central amygdala contributes to alcohol dependence-related anxiety-like behavior and excessive alcohol intake.
Serrano A, Pavon FJ, Buczynski MW, Schlosburg J, Natividad LA, Polis IY, Stouffer DG, Zorrilla EP, Roberto M, Cravatt BF, Martin-Fardon R, Rodriguez de Fonseca F, Parsons LH. Serrano A, et al. Neuropsychopharmacology. 2018 Aug;43(9):1840-1850. doi: 10.1038/s41386-018-0055-3. Epub 2018 Apr 5. Neuropsychopharmacology. 2018. PMID: 29748627 Free PMC article.
References
- Anderberg UM, Liu Z, Berglund L, Nyberg F. Plasma levels on nociceptin in female fibromyalgia syndrome patients. Z Rheumatol. 1998;57(Suppl 2):77–80. - PubMed
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. Functional heterogeneity in dopamine release and in the expression of Fos-like proteins within the rat striatal complex. Eur J Neurosci. 1999;11:1155–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous