Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn's disease. An in vivo and in vitro study - PubMed (original) (raw)
Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn's disease. An in vivo and in vitro study
Alessandra Zulian et al. PLoS One. 2013.
Abstract
Crohn's disease (CD) is notably characterized by the expansion of visceral fat with small adipocytes expressing a high proportion of anti-inflammatory genes. Conversely, visceral fat depots in ulcerative colitis (UC) patients have never been characterized. Our study aims were a) to compare adipocyte morphology and gene expression profile and bacterial translocation in omental (OM) and mesenteric (MES) adipose tissue of patients with UC and CD, and b) to investigate the effect of bacterial infection on adipocyte proliferation in vitro. Specimens of OM and MES were collected from 11 UC and 11 CD patients, processed and examined by light microscopy. Gene expression profiles were evaluated in adipocytes isolated from visceral adipose tissue using microarray and RTqPCR validations. Bacteria within adipose tissue were immuno-detected by confocal scanning laser microscopy. Adipocytes were incubated with Enterococcus faecalis and cells counted after 24 h. Morphology and molecular profile of OM and MES revealed that UC adipose tissue is less inflamed than CD adipose tissue. Genes linked to inflammation, bacterial response, chemotaxis and angiogenesis were down-regulated in adipocytes from UC compared to CD, whereas genes related to metallothioneins, apoptosis pathways and growth factor binding were up-regulated. A dense perinuclear positivity for Enterococcus faecalis was detected in visceral adipocytes from CD, whereas positivity was weak in UC. In vitro bacterial infection was associated with a five-fold increase in the proliferation rate of OM preadipocytes. Compared to UC, visceral adipose tissue from CD is more inflamed and more colonized by intestinal bacteria, which increase adipocyte proliferation. The influence of bacteria stored within adipocytes on the clinical course of IBD warrants further investigations.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
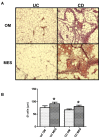
Figure 1. Morphological and morphometric characterization of adipose tissue from UC and CD patients.
A. Haematoxylin-eosin staining of omental (OM) and mesenteric adipose (MES) tissue sections from one representative ulcerative colitis (UC) and Crohn's disease (CD) patient. Magnification is 10X. B. Mean adipocyte size from different adipose tissue depots from 11 UC and 11 CD patients. Data are expressed as means ± SE, *P<0.05 between OM and MES in UC and CD patients.
Figure 2. Gene expression analysis of isolated adipocytes from UC and CD patients.
A. Clustering of global gene expression in omental adipose tissue (OM) and mesenteric adipose tissue around the involved intestinal tract (MES) in ulcerative colitis (UC) and Crohn's disease (CD) patients. B. Numbers of detected genes and percentage of differentially expressed genes in UC OM vs. CD OM and in UC MES vs. CD MES. Details of down- and up-regulated genes are indicated. C. Functional annotations of genes differentially expressed in UC OM vs. UC MES, in UC OM vs. CD OM and in UC MES vs. CD MES. GO: Gene Ontology-Biological Process. The first five most significant clusters of up- and down-regulated genes are shown.
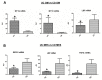
Figure 3. Validation of microarray data by Real Time quantitative PCR.
A. Expression of metallothionein 1G (MT1G), metallothionein 1E (MT1E), lipopolysaccharide-binding protein (LBP) mRNA in omental adipose tissue (OM) from Crohn's disease (CD) and ulcerative colitis (UC) patients. B. Expression of tumor necrosis factor alpha (TNFA), lipopolysaccharide-binding protein (LBP) and defensin beta 1 (DEF1B) in CD and UC MES. Data were normalized to human ribosomal protein LP0 (RPLP0) and are expressed as arbitrary units (AU) ± SE, *P<0.05.
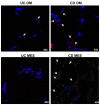
Figure 4. Detection of intestinal bacteria in visceral fat from UC and CD patients.
Fluorescent immunodetection of Enterococcus faecalis (red) in omental adipose tissue (OM) and mesenteric adipose tissue (MES) from 1 representative ulcerative colitis (UC) and 1 Crohn's disease (CD) patient by confocal microscopy. Magnification is 40X. Nuclei are counterstained in blue (DAPI, 4',6-diamidin-2-fenilindolo).
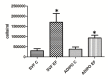
Figure 5. Effects of bacterial infection on adipocyte proliferation rate.
Mean cell number ± SE of omental preadipocytes (SVF) and 10-day differentiated adipocytes (ADIPO) from 4 patients (2UC and 2CD) after 24h incubation with (SVF EF and ADIPO EF) or without (SVF C and ADIPO C) 0.001 McFarland Enterococcus faecalis. *P<0.05.
Similar articles
- Visceral adiposity and inflammatory bowel disease.
Rowan CR, McManus J, Boland K, O'Toole A. Rowan CR, et al. Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review. - Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease.
Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C, Colombel JF, Desreumaux P. Peyrin-Biroulet L, et al. Gut. 2012 Jan;61(1):78-85. doi: 10.1136/gutjnl-2011-300370. Epub 2011 Sep 21. Gut. 2012. PMID: 21940721 Free PMC article. - Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease?
Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C. Zulian A, et al. Gut. 2012 Jan;61(1):86-94. doi: 10.1136/gutjnl-2011-300391. Epub 2011 Sep 19. Gut. 2012. PMID: 21930728 - T-cell Composition in Ileal and Colonic Creeping Fat - Separating Ileal from Colonic Crohn's Disease.
Kredel LI, Jödicke LJ, Scheffold A, Gröne J, Glauben R, Erben U, Kühl AA, Siegmund B. Kredel LI, et al. J Crohns Colitis. 2019 Jan 1;13(1):79-91. doi: 10.1093/ecco-jcc/jjy146. J Crohns Colitis. 2019. PMID: 30272118 - Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases.
Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Bilski J, et al. Biomolecules. 2019 Nov 26;9(12):780. doi: 10.3390/biom9120780. Biomolecules. 2019. PMID: 31779136 Free PMC article. Review.
Cited by
- Radiological biomarkers reflecting visceral fat distribution help distinguish inflammatory bowel disease subtypes: a multicenter cross-sectional study.
Xiong Z, Wu P, Zhang Y, Chen J, Shen Y, Kamel I, Wu B, Zheng X, Li Z. Xiong Z, et al. Insights Imaging. 2024 Mar 13;15(1):70. doi: 10.1186/s13244-024-01640-9. Insights Imaging. 2024. PMID: 38472526 Free PMC article. - Metabolic Disorders, the Microbiome as an Endocrine Organ, and Their Relations with Obesity: A Literature Review.
Ispas S, Tuta LA, Botnarciuc M, Ispas V, Staicovici S, Ali S, Nelson-Twakor A, Cojocaru C, Herlo A, Petcu A. Ispas S, et al. J Pers Med. 2023 Nov 13;13(11):1602. doi: 10.3390/jpm13111602. J Pers Med. 2023. PMID: 38003917 Free PMC article. Review. - Visceral Adiposity Independently Predicts Time to Flare in Inflammatory Bowel Disease but Body Mass Index Does Not.
Sehgal P, Su S, Zech J, Nobel Y, Luk L, Economou I, Shen B, Lewis JD, Freedberg DE. Sehgal P, et al. Inflamm Bowel Dis. 2024 Apr 3;30(4):594-601. doi: 10.1093/ibd/izad111. Inflamm Bowel Dis. 2024. PMID: 37307420 Free PMC article. - Anti-TNF Therapies Suppress Adipose Tissue Inflammation in Crohn's Disease.
Boronat-Toscano A, Monfort-Ferré D, Menacho M, Caro A, Bosch R, Espina B, Algaba-Chueca F, Saera-Vila A, Moliné A, Marti M, Espin E, Millan M, Serena C. Boronat-Toscano A, et al. Int J Mol Sci. 2022 Sep 22;23(19):11170. doi: 10.3390/ijms231911170. Int J Mol Sci. 2022. PMID: 36232469 Free PMC article. - Aspects Towards the Anastomotic Healing in Crohn's Disease: Clinical Approach and Current Gaps in Research.
Chaim FHM, Negreiros LMV, Steigleder KM, Siqueira NSN, Genaro LM, Oliveira PSP, Martinez CAR, Ayrizono MLS, Fagundes JJ, Leal RF. Chaim FHM, et al. Front Surg. 2022 Jun 24;9:882625. doi: 10.3389/fsurg.2022.882625. eCollection 2022. Front Surg. 2022. PMID: 35813046 Free PMC article. Review.
References
- Bamias G, Pizarro T, Cominelli F (2011) New Paradigms in the Pathogenesis of IBD. In: Cohen RD, Inflammatory Bowel Disease: Diagnosis and Therapeutics. pp. 41-57.
Publication types
MeSH terms
Grants and funding
This study was supported by Istituto Auxologico Italiano. Istituto Auxologico Italiano, funder of this study, had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous