Non-canonical Hedgehog signaling contributes to chemotaxis in cholangiocarcinoma - PubMed (original) (raw)
Non-canonical Hedgehog signaling contributes to chemotaxis in cholangiocarcinoma
Nataliya Razumilava et al. J Hepatol. 2014 Mar.
Erratum in
- J Hepatol. 2014 Jul;61(1):185
Abstract
Background & aims: The Hedgehog signaling pathway contributes to cholangiocarcinoma biology. However, canonical Hedgehog signaling requires cilia, and cholangiocarcinoma cells often do not express cilia. To resolve this paradox, we examined non-canonical (G-protein coupled, pertussis toxin sensitive) Hedgehog signaling in cholangiocarcinoma cells.
Methods: Human [non-malignant (H69), malignant (HuCC-T1 and Mz-ChA-1)] and rat [non-malignant (BDE1 and NRC), and malignant (BDEneu)] cell lines were employed for this study. A BDE(ΔLoop2) cell line with the dominant-negative receptor Patched-1 was generated with the Sleeping Beauty transposon transfection system.
Results: Cilia expression was readily identified in non-malignant, but not in malignant cholangiocarcinoma cell lines. Although the canonical Hh signaling pathway was markedly attenuated in cholangiocarcinoma cells, they were chemotactic to purmorphamine, a small-molecule direct Smoothened agonist. Purmorphamine also induced remodeling of the actin cytoskeleton with formation of filopodia and lamellipodia-like protrusions. All these biological features of cell migration were pertussis toxin sensitive, a feature of G-protein coupled (Gis) receptors. To further test the role of Hedgehog signaling in vivo, we employed a syngeneic orthotopic rat model of cholangiocarcinoma. In vivo, genetic inhibition of the Hedgehog signaling pathway employing BDE(ΔLoop2) cells or pharmacological inhibition with a small-molecule antagonist of Smoothened, vismodegib, was tumor and metastasis suppressive.
Conclusions: Cholangiocarcinoma cells exhibit non-canonical Hedgehog signaling with chemotaxis despite impaired cilia expression. This non-canonical Hedgehog signaling pathway appears to contribute to cholangiocarcinoma progression, thereby, supporting a role for Hedgehog pathway inhibition in human cholangiocarcinoma.
Keywords: 4′-6-diamidino-2-phenylindole; Biliary tract cancer; CAFs; CCA; DAPI; Dominant-negative Ptch1; FBS; G-protein coupled receptor; Gli; Hedgehog; Hh; PBS; PTX; Patched-1; Ptch1; Shh; Smo; Smoothened; Sonic Hh; cancer-associated fibroblasts; cholangiocarcinoma; fetal bovine serum; glioma-associated transcriptional factor; pertussis toxin; phosphate-buffered saline; qRT-PCR; quantitative reverse transcription polymerase chain reaction.
Copyright © 2013 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures
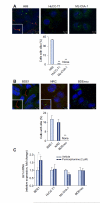
Fig. 1. CCA cells have impaired cilium expression and do not display canonical Hh signaling activation.
(A) Human non-malignant (H69) and CCA (HuCC-T1 and Mz-ChA-1) cells and (B) rat non-malignant (BDE1 and NRC) and CCA (BDEneu) cells were examined by confocal microscopy for immunofluorescence for acetylated α- and γ-tubulin, markers for cilia (red) and centromere (green) expression (A and B, top) respectively. Slides were analyzed under direct visualization, and results are presented as a percent of cells possessing cilia from the total number of cells in a high power microscopy field (A and B, bottom; mean ± SEM; **p <0.01). (C) Non-malignant (BDE1) and malignant (HuCC-T1, MzChA-1, and BDEneu) cell lines were cultured and treated with a small-molecule agonist of Smo, purmorphamine, at 2 μM for 72 h. Total RNA was then subjected to qRT-PCR for Gli1 as well as 18S (internal control) mRNA expression. Relative expression was determined (Δ-Δ CT compared to 18S), and results are presented as a fold change in Gli1 mRNA expression in the purmorphamine treated cells as compared to the vehicle treated cells (mean ± SEM; *p <0.05). (This figure appears in colour on the web.)
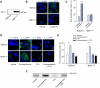
Fig. 2. The Hh signaling pathway is activated in CCA cells.
(A) BDEneu cells were genetically modified to express dominant-negative Ptch1. Genomic DNA was isolated from BDEneu and BDEΔLoop2 cells and examined on an agarose gel for presence of Ptch1 lacking second extracellular loop (PtchΔLoop2). The apparent DNA size is indicated in number of base pairs (bp). (B) The BDEneu and BDEΔLoop2 cells were plated and treated with either vehicle or rm-Shh-N (6 μM). Cells were examined by confocal microscopy for Smo immunofluorescence (green). (C) Slides were analyzed and cells counted using ImageJ software; results are presented as percent of cells with Smo expression at the plasma membrane from the total number of cells per a high power field (mean ± SEM; ***p <0.001). (D) The BDEneu and BDEΔLoop2 cells were plated and treated with either vehicle or purmorphamine (2 μM) with and without PTX (200 μg/ml) for 16 h. Cells were examined by confocal microscopy for Smo immunofluorescence (green) and Smo localization to the plasma membrane. (E) Slides were analyzed and cells counted using ImageJ software; results are presented as percent of cells with Smo expression at the plasma membrane from the total number of cells per a high power field (mean ± SEM; ***p <0.001). (F) BDEneu cells were cultured and treated with either vehicle or purmorphamine (2 μM) with and without PTX (200 μg/ml) for 16 h. Surface proteins were biotinylated, purified, and analyzed for Smo protein expression with immunoblot. Apparent molecular weight is indicated in kDa. (This figure appears in colour on the web.)
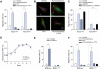
Fig. 3. In CCA cells, activation of the Hh signaling pathway requires Ptch1 and leads to cell migration with cytoskeleton rearrangements in a G-protein dependent manner.
Modified Boyden chambers were employed to study cell migration (A, C, E, and F). Membranes were prepared, and cell nuclei were marked with DAPI. Cell migration across the membrane was assessed with direct visualization using fluorescence microscopy. Due to differences in migratory biology, human and rat CCA cell lines were treated over different time periods. Results are presented as a percent of migrated cells from the total number of cells. (A) The human CCA (HuCC-T1 and Mz-ChA-1) cells were studied for chemotaxis by vehicle and purmorphamine (2 μM) with and without PTX (200 μg/ml) for 8 h (mean ± SEM; **p <0.01). (B) The CCA cell line was studied for the actin cytoskeleton remodeling and localization of the focal adhesion-associated protein, paxillin. The HuCC-T1 cells were treated with either vehicle or purmorphamine (2 μM) with and without PTX (200 μg/ml) for 8 h and examined under the fluorescence microscope for phalloidin (red) marking F-actin expression and paxillin (green) marking focal adhesions (arrows). Results are shown as representative images taken at resolution of 630×. (C) The human CCA (HuCC-T1 and Mz-ChA-1) cells were studied for chemotaxis by vehicle or GANT61 (20 μM) with and without purmorphamine (2 μM) for 8 h (mean ± SEM; **p <0.01). (D) The rat CCA cells (BDEneu and BDEΔLoop2) were cultured and examined daily with colorimetric assay for the cell proliferation rate. Results are presented as an absorbance at 480 nm wavelength (reference wavelength is 630 nm). (E, F) The rat CCA cells (BDEneu and BDEΔLoop2) were studied for chemotaxis by vehicle, rm-Shh-N (6 μM; E), or purmorphamine (2 μM; F) with or without PTX (200 μg/ml; F) for 24 h (mean ± SEM; *p <0.05; ***p <0.001). (This figure appears in colour on the web.)
Fig. 4. In vivo, genetic and pharmacological inhibition of the Hh pathway is tumor and metastases suppressive.
A syngeneic orthotopic rat CCA model was employed for the experiments (Fisher 344 rats; A–D). (A, B) 21 days after CCA cells (BDEneu and BDEΔLoop2) implantation, animals were euthanized and examined for the presence of tumor and tumor metastases. (A) Depicted are representative explanted livers from the animals implanted with either BDEneu (n = 9; left) or BDEΔLoop2 (n = 9; right) cells. (B) A stacked column plot represents a percent of animals with tumor with and without metastases (mean ± SEM; *p <0.05). (C, D) Animals were implanted with BDEneu cells and treated with either vehicle (n = 10) or vismodegib (n = 11; 25 mg/kg, intraperitoneally; day 0–6). Animals were euthanized on day 7 and examined for the presence of tumors (C) and liver and tumor weight (D). Results are presented as a percent of animals that developed tumor (C) and as a percent of tumor weight from the total liver weight (D; mean ± SEM; *p <0.05; n.s., non significant). (E, F) Animals were implanted with BDEneu cells and treated with either vehicle (n = 9) or vismodegib (n = 10; 25 mg/kg; intraperitoneally; day 7–21). Animals were euthanized on day 22 and examined for tumor (E) and metastases burden (F). Results for tumor burden are presented as a percent of tumor weight from total liver weight (E; mean ± SEM; *p <0.05). Metastases burden is represented as an average metastases burden [size of metastases for each examined site (omentum, peritoneum, and diaphragm) on a scale from 0 to 4] per animal in vehicle and vismodegib treated groups (mean ± SEM; **p <0.01). (G) A schematic diagram illustrating the role of the non-canonical Hh signaling pathway in CCA. (This figure appears in colour on the web.)
Similar articles
- The intrahepatic signalling niche of hedgehog is defined by primary cilia positive cells during chronic liver injury.
Grzelak CA, Martelotto LG, Sigglekow ND, Patkunanathan B, Ajami K, Calabro SR, Dwyer BJ, Tirnitz-Parker JE, Watkins DN, Warner FJ, Shackel NA, McCaughan GW. Grzelak CA, et al. J Hepatol. 2014 Jan;60(1):143-51. doi: 10.1016/j.jhep.2013.08.012. Epub 2013 Aug 23. J Hepatol. 2014. PMID: 23978713 - Polo-like kinase 2 is a mediator of hedgehog survival signaling in cholangiocarcinoma.
Fingas CD, Mertens JC, Razumilava N, Sydor S, Bronk SF, Christensen JD, Ilyas SI, Canbay A, Treckmann JW, Paul A, Sirica AE, Gores GJ. Fingas CD, et al. Hepatology. 2013 Oct;58(4):1362-74. doi: 10.1002/hep.26484. Epub 2013 Aug 6. Hepatology. 2013. PMID: 23703673 Free PMC article. - Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells.
Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, Mertens JC, Sirica AE, Gores GJ. Fingas CD, et al. Hepatology. 2011 Dec;54(6):2076-88. doi: 10.1002/hep.24588. Hepatology. 2011. PMID: 22038837 Free PMC article. - Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins.
Polizio AH, Chinchilla P, Chen X, Manning DR, Riobo NA. Polizio AH, et al. Sci Signal. 2011 Nov 22;4(200):pt7. doi: 10.1126/scisignal.2002396. Sci Signal. 2011. PMID: 22114142 Free PMC article. Review. - Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened.
Pietrobono S, Gagliardi S, Stecca B. Pietrobono S, et al. Front Genet. 2019 Jun 12;10:556. doi: 10.3389/fgene.2019.00556. eCollection 2019. Front Genet. 2019. PMID: 31244888 Free PMC article. Review.
Cited by
- The hedgehog pathway in triple-negative breast cancer.
Habib JG, O'Shaughnessy JA. Habib JG, et al. Cancer Med. 2016 Oct;5(10):2989-3006. doi: 10.1002/cam4.833. Epub 2016 Aug 18. Cancer Med. 2016. PMID: 27539549 Free PMC article. Review. - Role of Hedgehog Signaling Pathway in NASH.
Verdelho Machado M, Diehl AM. Verdelho Machado M, et al. Int J Mol Sci. 2016 Jun 1;17(6):857. doi: 10.3390/ijms17060857. Int J Mol Sci. 2016. PMID: 27258259 Free PMC article. Review. - The Tumor Immune Microenvironment plays a Key Role in Driving the Progression of Cholangiocarcinoma.
Zhang Y, Yan HJ, Wu J. Zhang Y, et al. Curr Cancer Drug Targets. 2024;24(7):681-700. doi: 10.2174/0115680096267791231115101107. Curr Cancer Drug Targets. 2024. PMID: 38213139 Review. - Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments.
Jeng KS, Chang CF, Lin SS. Jeng KS, et al. Int J Mol Sci. 2020 Jan 23;21(3):758. doi: 10.3390/ijms21030758. Int J Mol Sci. 2020. PMID: 31979397 Free PMC article. Review. - Selecting an Appropriate Experimental Animal Model for Cholangiocarcinoma Research.
Li M, Zhou X, Wang W, Ji B, Shao Y, Du Q, Yao J, Yang Y. Li M, et al. J Clin Transl Hepatol. 2022 Aug 28;10(4):700-710. doi: 10.14218/JCTH.2021.00374. Epub 2022 Feb 11. J Clin Transl Hepatol. 2022. PMID: 36062286 Free PMC article. Review.
References
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. - PubMed
- El Khatib M, Kalnytska A, Palagani V, Kossatz U, Manns MP, Malek NP, et al. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology. 2013;57:1035–1045. - PubMed
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007198/DK/NIDDK NIH HHS/United States
- R01 DK059427/DK/NIDDK NIH HHS/United States
- R56 DK059427/DK/NIDDK NIH HHS/United States
- DK59427/DK/NIDDK NIH HHS/United States
- R21CA166635/CA/NCI NIH HHS/United States
- R01 CA083650/CA/NCI NIH HHS/United States
- R21 CA166635/CA/NCI NIH HHS/United States
- P30DK084567/DK/NIDDK NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- CA83650/CA/NCI NIH HHS/United States
- P30 DK084567/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous