IL-10 inhibits the NF-κB and ERK/MAPK-mediated production of pro-inflammatory mediators by up-regulation of SOCS-3 in Trypanosoma cruzi-infected cardiomyocytes - PubMed (original) (raw)
IL-10 inhibits the NF-κB and ERK/MAPK-mediated production of pro-inflammatory mediators by up-regulation of SOCS-3 in Trypanosoma cruzi-infected cardiomyocytes
Eugenia Hovsepian et al. PLoS One. 2013.
Abstract
Trypanosoma cruzi (T. cruzi) infection produces an intense inflammatory response which is critical for the control of the evolution of Chagas' disease. Interleukin (IL)-10 is one of the most important anti-inflammatory cytokines identified as modulator of the inflammatory reaction. This work shows that exogenous addition of IL-10 inhibited ERK1/2 and NF-κB activation and reduced inducible nitric oxide synthase (NOS2), metalloprotease (MMP) -9 and MMP-2 expression and activities, as well as tumour necrosis factor (TNF)-α and interleukin (IL)-6 expression, in T. cruzi-infected cardiomyocytes. We found that T. cruzi and IL-10 promote STAT3 phosphorylation and up-regulate the expression of suppressor of cytokine signalling (SOCS)-3 thereby preventing NF-κB nuclear translocation and ERK1/2 phosphorylation. Specific knockdown of SOCS-3 by small interfering RNA (siRNA) impeded the IL-10-mediated inhibition of NF-κB and ERK1/2 activation. As a result, the levels of studied pro-inflammatory mediators were restored in infected cardiomyocytes. Our study reports the first evidence that T. cruzi up- regulates SOCS-3 expression and highlights the relevance of IL-10 in the modulation of pro-inflammatory response of cardiomyocytes in Chagas' disease.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. IL-10 inhibits cardiomyocytes inflammatory response induced by T. cruzi infection.
(A) Cardiomyocytes were pre-treated with IL-10 (10 ng/ml or 20 ng/ml) for 15 min before T. cruzi infection (with parasite:cell ratio 5∶1) and NO levels were quantified by Griess reaction in the culture media after 48 h (n = 3). (B) Neonatal cardiac cells were infected for 10 and 20 min or pre-treated for 15 min with 20 ng/ml of IL-10 and cytosolic extracts were prepared. The levels of P-ERK1/2-MAPK were determined by Wb with specific antibodies. (C) Cells were pre-treated with IL-10 in the same conditions as in B and infected with T. cruzi during 30 or 120 minutes for p65 nuclear detection or (D) pre-treated with IL-10 and infected for 15 or 30 min for cytosolic detection of IκB-α. For NO measurement, results are expressed as mean±SD (n = 4). For B, C and D results show a representative experiment of three performed and protein levels were normalized against α-actin (B and D) and Sp1 (C). Mean±SD (n = 3). **p<0.01 vs. control cells; #p<0.05 vs. T. cruzi infected cells.
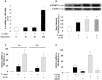
Figure 2. T. cruzi and IL-10 promote STAT3 activation and SOCS-3 expression.
(A) Kinetics of IL-6 transcription after T. cruzi infection of cardiomyocytes. Cells were infected with T. cruzi trypomastigotes and at different time points mRNA for IL-6 was assessed using Q-RT-PCR. (B) Cells were infected or treated with IL-10 (20 ng/ml) or pre-treated with IL-10 and infected for 30 min and STAT3 activation was analysed by Wb. (C) Neonatal cardiomyocytes were infected or treated with IL-10 (20 ng/ml) for 1 or 2 h, and SOCS-3 mRNA levels were analysed by Q-RT-PCR. (D) Cells were pre-incubated with STAT3 inhibitor, stattic (10 µM), for 30 min before 1 h of T. cruzi infection or IL-10 treatment and SOCS-3 mRNA levels were analysed by Q-RT-PCR. For A, C and D, results were normalized against 18S and represent the mean±SD of three independent experiments. For B, results show the mean±SD (n = 3) and protein levels were normalized against α-actin. **p<0.01 vs. control cells; *p<0.05 vs. control cells; ˆp<0.01 vs. T. cruzi infected cells; εp<0.01 vs. IL-10 treated cells.
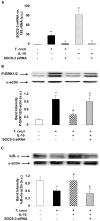
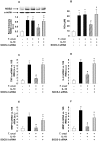
Figure 3. SOCS-3 is involved in the inhibition of ERK1/2-MAPK and NF-κB after IL-10 treatment of infected cardiomyocytes.
(A) Cells were transfected with SOCS-3 siRNA for 72 h, infected with T. cruzi or treated with IL-10 for 1 h and SOCS-3 mRNA levels were analysed by Q-RT-PCR. (B) Cells were infected with T. cruzi, pre-treated with IL-10 or transfected with SOCS-3 siRNA for 72 h or pre-treated with IL-10 and infected for 20 min. P-ERK1/2-MAPK levels were determined by Wb with specific antibodies. (C) Cytosolic extracts from cardiomyocytes in the same conditions as in B but infected for 30 min were analysed by Wb to determine IκB-α expression. For A, results were normalized against 18S and represent the mean±SD of three independent experiments. For B and C, results show the mean±SD (n = 3) and protein levels were normalized against α-actin. **p<0.01 vs. control cells; ˆp<0.01 vs. T. cruzi infected cells; #p<0.05 vs. T. cruzi infected cells; εp<0.01 vs. IL-10 treated cells; φp<0.05 vs. T. cruzi infected+IL-10 treated cells.
Figure 4. IL-10 treatment inhibits pro-inflammatory cytokines and NOS2 expression and activity in T. cruzi infected cardiomyocytes.
(A) Cells were infected with T. cruzi or pre-treated with IL-10 (20 ng/ml) and TNF-α and IL-6 mRNA levels were analysed by Q-RT-PCR 4 h post infection. (B) NOS2 expression was analysed by Wb in total protein extracts obtained after 48 h of T. cruzi infection or after pre-treatment with IL-10. (C) Cells were grown on round glass cover slips and infected with T. cruzi or pre-treated with 20 ng/ml of IL-10 for 15 min. After 48 h of infection, NO synthesis was detected in situ by the NO indicator DAF-FM and visualized by fluorescence microscopy. For A, results were normalized against 18S and represent the mean±SD of three independent experiments. For B, results show the mean±SD (n = 4) and protein levels were normalized against α-actin. In C, microphotographs (400X) are representative of three independent experiments performed. The graph represents the mean±SD of the integrated fluorescence densities. **p<0.01 vs. control cells; ˆp<0.01 vs. T. cruzi infected cells; #p<0.05 vs. T. cruzi infected cells.
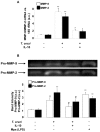
Figure 5. Effect of IL-10 treatment on cardiac matrix metalloproteinase (MMP)-9 and MMP-2 expression and activity after T. cruzi infection.
(A) Neonatal cardiomyocytes were treated with IL-10 (20 ng/ml) for 15 min followed by 48 h of T. cruzi infection. MMP-9 and MMP-2 mRNA levels were analysed by Q-RT-PCR. (B) Under the same conditions as in A, zymograms of MMP-9 and MMP-2 activity were carried out in culture media of neonatal cardiomyocytes after 72 h of T. cruzi infection. White bars indicate the values of MMP-9 activity and black bars indicate the values of MMP-2 activity. Peritoneal macrophages (Mps) were stimulated with LPS for 24 hours and used as a positive control for MMP. In A, the results were normalized against 18S and represent the mean±SD of three independent experiments. For B, results represent the relative densitometric analysis of the activities of Pro-MMP-9 and Pro-MMP-2 of four experiments performed. **p<0.01 vs. Control cells; ˆp<0.01, #p<0.05 vs. T. cruzi infected cells.
Figure 6. SOCS-3 is involved in the anti-inflammatory effects exerted by IL-10.
Cardiomyocytes were pre-treated with 20 ng/ml of IL-10, infected with T. cruzi or transfected with SOCS-3 siRNA for 72 h, pre-treated with IL-10 and infected for 48 h. (A) NOS2 expression levels were determined by Wb. (B) In the same conditions NOS2 activity was evaluated by quantification of NO levels by Griess reaction in the culture media. (C) and (D) TNF-α and IL-6 mRNA levels were analysed by Q-RT-PCR in the same conditions but only for 4 h post infection. (E) and (F) In the same conditions as in (A), MMP-9 and MMP-2 mRNA levels were analysed by Q-RT-PCR. For A, results show the mean±SD (n = 3) and protein levels were normalized against α-actin. In B, results are expressed as mean±SD (n = 3). In C,D,E and F, the results were normalized against 18S and represent the mean±SD of three independent experiments **p<0.01 vs. control cells; #p<0.05 vs. T. cruzi infected cells; φp<0.05 vs. T. cruzi infected+IL-10 treated cells.
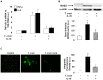
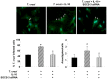
Figure 7. SOCS-3 silencing reverts IL-10-induced cardiomyocytes parasitism.
Cells grown on glass coverslips were infected, infected and treated with 20/ml of IL-10 or silenced by siRNA for 72 h and then pre-treated with IL-10 and infected for 48 h. Infected cells were incubated with rabbit polyclonal serum against to T. cruzi followed by FITC-labeled goat anti-rabbit IgG. Afterwards, cells were counterstained with DAPI (300 nM). The percentage of infected cells (left bar graph) and the number of amastigotes/cell (right bar graph) are shown. Results represent the Mean ± SD of two experiments. Microphotographs are representative of 30 fields taken at 400X magnification. White arrows show FITC-anti-_T.cruzi-_labelled intracellular amastigotes.
Similar articles
- Modulation of inflammatory response and parasitism by 15-Deoxy-Δ(12,14) prostaglandin J(2) in Trypanosoma cruzi-infected cardiomyocytes.
Hovsepian E, Mirkin GA, Penas F, Manzano A, Bartrons R, Goren NB. Hovsepian E, et al. Int J Parasitol. 2011 Apr;41(5):553-62. doi: 10.1016/j.ijpara.2010.12.002. Epub 2011 Jan 6. Int J Parasitol. 2011. PMID: 21215746 - PPARγ ligand treatment inhibits cardiac inflammatory mediators induced by infection with different lethality strains of Trypanosoma cruzi.
Penas F, Mirkin GA, Hovsepian E, Cevey A, Caccuri R, Sales ME, Goren NB. Penas F, et al. Biochim Biophys Acta. 2013 Jan;1832(1):239-48. doi: 10.1016/j.bbadis.2012.08.007. Epub 2012 Aug 16. Biochim Biophys Acta. 2013. PMID: 22917565 - Trypanosoma cruzi induces the reactive oxygen species-PARP-1-RelA pathway for up-regulation of cytokine expression in cardiomyocytes.
Ba X, Gupta S, Davidson M, Garg NJ. Ba X, et al. J Biol Chem. 2010 Apr 9;285(15):11596-606. doi: 10.1074/jbc.M109.076984. Epub 2010 Feb 9. J Biol Chem. 2010. PMID: 20145242 Free PMC article. - α₁ adrenoceptor activation by norepinephrine inhibits LPS-induced cardiomyocyte TNF-α production via modulating ERK1/2 and NF-κB pathway.
Yu X, Jia B, Wang F, Lv X, Peng X, Wang Y, Li H, Wang Y, Lu D, Wang H. Yu X, et al. J Cell Mol Med. 2014 Feb;18(2):263-73. doi: 10.1111/jcmm.12184. Epub 2013 Dec 5. J Cell Mol Med. 2014. PMID: 24304472 Free PMC article. - Low-dose benznidazole treatment results in parasite clearance and attenuates heart inflammatory reaction in an experimental model of infection with a highly virulent Trypanosoma cruzi strain.
Cevey ÁC, Mirkin GA, Penas FN, Goren NB. Cevey ÁC, et al. Int J Parasitol Drugs Drug Resist. 2015 Dec 12;6(1):12-22. doi: 10.1016/j.ijpddr.2015.12.001. eCollection 2016 Apr. Int J Parasitol Drugs Drug Resist. 2015. PMID: 26862474 Free PMC article.
Cited by
- The evolving landscape of IL-10, IL-22 and IL-26 in pleurisy especially in tuberculous pleurisy.
Niu Q, Wang M, Liu XS. Niu Q, et al. Respir Res. 2024 Jul 13;25(1):275. doi: 10.1186/s12931-024-02896-x. Respir Res. 2024. PMID: 39003443 Free PMC article. Review. - Expression Analysis of the Mediators of Epithelial to Mesenchymal Transition and Early Risk Assessment of Therapeutic Failure in Laryngeal Carcinoma.
Kariche N, Moulaï N, Sellam LS, Benyahia S, Ouahioune W, Djennaoui D, Touil-Boukoffa C, Bourouba M. Kariche N, et al. J Oncol. 2019 Dec 6;2019:5649846. doi: 10.1155/2019/5649846. eCollection 2019. J Oncol. 2019. PMID: 31885577 Free PMC article. - Catabolic and proinflammatory effects of leptin in chondrocytes are regulated by suppressor of cytokine signaling-3.
Koskinen-Kolasa A, Vuolteenaho K, Korhonen R, Moilanen T, Moilanen E. Koskinen-Kolasa A, et al. Arthritis Res Ther. 2016 Oct 3;18(1):215. doi: 10.1186/s13075-016-1112-0. Arthritis Res Ther. 2016. PMID: 27716333 Free PMC article. - Attenuation of Inflammatory Mediators (TNF-α and Nitric Oxide) and Up-Regulation of IL-10 by Wild and Domesticated Basidiocarps of Amauroderma rugosum (Blume & T. Nees) Torrend in LPS-Stimulated RAW264.7 Cells.
Chan PM, Tan YS, Chua KH, Sabaratnam V, Kuppusamy UR. Chan PM, et al. PLoS One. 2015 Oct 1;10(10):e0139593. doi: 10.1371/journal.pone.0139593. eCollection 2015. PLoS One. 2015. PMID: 26427053 Free PMC article. - Downregulation of inflammatory erectile dysfunction by Mantisa religiosa egg-cake through NO-cGMP-PKG dependent NF-kB signaling cascade activated by mixture of salt intake.
Akintunde JK, Olayinka MC, Ugbaja VC, Akinfenwa CA, Akintola TE, Akamo AJ, Bello IJ. Akintunde JK, et al. Toxicol Rep. 2023 May 20;10:633-646. doi: 10.1016/j.toxrep.2023.05.007. eCollection 2023. Toxicol Rep. 2023. PMID: 37250529 Free PMC article.
References
- Abrahamsohn IA, Coffman RL (1995) Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 155: 3955–3963. - PubMed
- Hovsepian E, Mirkin GA, Penas F, Manzano A, Bartrons R, et al. (2011) Modulation of inflamatory response and parasitism by 15-Deoxy-Δ12,14 prostaglandin J(2) in Trypanosoma cruzi-infected cardyomyocites. Int J Parasitol. 41: 553–562. - PubMed
- Penas F, Mirkin GA, Hovsepian E, Cevey A, Caccuri R, et al. (2013) PPARγ ligand treatment inhibits cardiac inflammatory mediators induced by infection with different lethality strains of Trypanosoma cruzi. Biochim Biophys Acta. 1832: 239–248. - PubMed
- Borges MM, Kloetzel JK, Andrade HF Jr, Tadokoro CE, Pinge-Filho P, et al. (1998) Prostaglandin and nitric oxide regulate TNF-alpha production during Trypanosoma cruzi infection. Immunol Lett. 63: 1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by Grants PICT 2007 N° 995 from Agencia Nacional de Promoción de Ciencia y Tecnología (ANPCyT) Argentina and PIP 1424 from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Argentina and UBACyT 20020100100809 from Universidad de Buenos Aires, Buenos Aires, Argentina. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous