The effect of 3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole (YC-1) on cell viability under hypoxia - PubMed (original) (raw)
. 2013 Nov 16:19:2260-73.
eCollection 2013.
Affiliations
- PMID: 24265542
- PMCID: PMC3834593
The effect of 3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole (YC-1) on cell viability under hypoxia
Leo Tsui et al. Mol Vis. 2013.
Abstract
Purpose: The synthetic compound 3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole (YC-1) reduces the protein stability of hypoxia-inducible factor (HIF)-1α and can serve as a potential anticancer agent. Our previous study elucidated that YC-1 decreased the protein level of HIF-1α and inhibited cell proliferation under normoxic conditions. In the present study, we explored the inhibitory effect of YC-1 on the regulation of HIF-1α and cell survival under hypoxia.
Methods: Chemical and physical hypoxia using cobalt chloride and an anaerobic incubator, respectively, was induced in the photoreceptor cell line 661W. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and morphological observation were used to analyze cell survival. Flow cytometry with a LIVE/DEAD cell viability assay and annexin V was used to determine the number of live and dead cells or cell apoptosis, respectively. Cell proliferation was analyzed with high-content screening of MKI67 (K(i)-67) immunofluorescent staining. Immunoblotting and a quantitative reverse-transcription PCR were used to assess the protein and mRNA levels, respectively.
Results: Our results showed that 661W cells exposed to YC-1 decreased cell survival through the induction of cell apoptosis and cell-cycle arrest under hypoxia. We also found that YC-1 reduced the HIF-1α protein level after 2 h of hypoxia, but the mRNA level of HIF-1α was not affected. In addition, YC-1 significantly increased levels of p53, the proapoptotic gene BCL2-associated X protein (Bax), and cell proliferation-related gene, cyclin-dependent kinase inhibitor 1A (p21) mRNAs under hypoxia.
Conclusions: Unlike normoxia, YC-1 not only inhibited cell proliferation but also induced cell death under hypoxia. We also found that YC-1 inhibited hypoxia-induced HIF-1α and partially affected hypoxia-regulated gene expression.
Figures
Figure 1
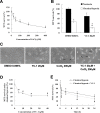
3-(5′-hydroxymethyl-2’-furyl)-1-benzylindazole (YC-1) decreased cell survival in a dose- and time-related manner under chemical hypoxia. A: 661W cells were treated with 25, 50, 100, 200, and 400 μM CoCl2 for 24 h, and cell viability was measured with an MTT assay (n=4). B: The viability of 661W cells in the presence of 0.066% DMSO or 20 μM YC-1 for 24 h during chemically induced hypoxia (by 200 μM CoCl2) was evaluated using an MTT assay (n=4). C: Morphological changes in cell density were observed with light microscopy. The black arrows indicate the cell debris. D: Concentration-dependent reduction in cell survival by YC-1 during chemical hypoxia. 661W cells were exposed to 5, 10, 20, and 40 μM YC-1 for 5 min, followed by 200 μM CoCl2 for 24 h (n=4). E: Time-dependent curves of cell survival in response to YC-1 in hypoxia. 661W cells were incubated with 200 μM CoCl2 in the absence or presence of 20 μM YC-1 for 1, 4, 12, and 24 h (n=4). * Indicates p<0.05 compared to the control group or the DMSO vehicle group; # indicates p<0.05 compared to the chemically induced hypoxic group.
Figure 2
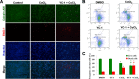
LIVE/DEAD cell viability assay of YC-1-treated cells under chemical hypoxia. A: After treatment with 200 μM CoCl2 in the absence or presence of 20 μM YC-1 for 24 h, 661W cells were stained with calcein AM (green for live cells), EthD-1 (red for dead cells), and Hoechst 33,342 (blue for nuclei). Representative fluorescence staining shows the cell density and composition. B: 661W cells were exposed to 0.066% DMSO, 20 μM YC-1, 200 μM CoCl2, or both YC-1 and CoCl2 for 24 h. After fluorescence staining, cells was measured and analyzed with flow cytometry. C: Quantitative data of flow cytometric analysis are from three independent experiments (n=3). * Indicates p<0.05 compared to the control group or DMSO vehicle group; # indicates p<0.05 compared to the chemically induced hypoxic group.
Figure 3
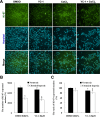
High-content screening (HCS) of YC-1-treated cells under chemical hypoxia. 661W cells were exposed to 0.066% DMSO, 20 μM YC-1, 200 μM CoCl2, or both YC-1 and CoCl2 for 24 h. After fixation and immunofluorescence staining, cells were detected and analyzed using a Cellomics ArrayScan VTI HCS Reader. A: Representative fluorescent photographs show the distribution of Ki-67 (green) and Hoechst-labeled nuclei (blue) using the Columbus Image Data Storage and Analysis System. Panels (B) and (C) show quantified HCS results for the number and ratio of Ki-67-stained nuclei (n=4). * Indicates p<0.05 compared to the control group or the DMSO vehicle group; # indicates p<0.05 compared to the chemically induced hypoxic group.
Figure 4
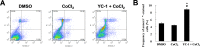
YC-1 increased cell apoptosis under chemical hypoxia. A: After treatment with 0.066% DMSO or 200 µM CoCl2 in the absence or presence of 20 µM YC-1 for 24 h, 661W cells were stained with annexin V (for apoptotic cells) and propidium iodide (for necrotic cells) and measured and analyzed by flow cytometry. B: The frequency of apoptosis was quantified from three independent experiments (n=3). * Indicates p<0.05 compared to DMSO vehicle group; # indicates p<0.05 compared to the chemically induced hypoxic group.
Figure 5
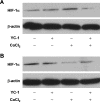
Effect of YC-1 on HIF-1α protein expression under chemical hypoxia. Total proteins were extracted from 661W cells incubated with 0.066% DMSO, 20 µM YC-1, 200 µM CoCl2, or both YC-1 and CoCl2 for 2 h (A) and 24 h (B). Expressions of HIF-1α and β-actin (which served as the internal control) were determined with immunoblotting.
Figure 6
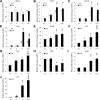
Quantitative reverse-transcription PCR (qRT-PCR) analysis of related genes in response to YC-1 under chemical hypoxia. 661W cells were incubated with 0.066% DMSO, 20 μM YC-1, 200 μM CoCl2, or both YC-1 and CoCl2 for 2 h or 24 h. After mRNA was extracted and reverse transcription was performed, relative HIF-1α (A), VEGF (B), glucose transporter 1 (GLUT1; C), carbonic anhydrase 9 (Car9; D), NF-κB (E), p53 (F), Bax (G), Bcl2 (H), Apaf1 (I), and cyclin-dependent kinase inhibitor 1A (p21; J) mRNAs were measured and compared to β-actin mRNA with qRT-PCR (n=3). * Indicates p<0.05 compared to the control group or the DMSO vehicle group; # indicates p<0.05 compared to the chemically induced hypoxic group; + indicates p<0.05 compared to the YC-1-treated group. D, DMSO; Y, YC-1; C, CoCl2; Y+C, both YC-1 and CoCl2.
Figure 7
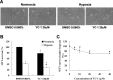
YC-1 reduced cell survival under physical hypoxia. A: 661W cells were exposed to 0.066% DMSO or 20 μM YC-1 for 24 h under normoxia or hypoxia (0.5% O2, 5% CO2). Morphological changes in cell density were observed with light microscopy. Panel (B) is the quantified cell viability determined with an MTT assay (n=4). Panel (C) shows a concentration-dependent cell survival curve of YC-1 during hypoxia (n=4). 661W cells were incubated with 5, 10, 20, and 40 μM YC-1 for 5 min, followed by exposure to physical hypoxia for 24 h. * Indicates p<0.05 compared to the control group or the DMSO vehicle group; # indicates p<0.05 compared to the hypoxic group.
Figure 8
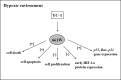
Summary of the effects of YC-1 on 661W cell viability under hypoxia.
Similar articles
- Effects of YC-1 on hypoxia-inducible factor 1 alpha in hypoxic human bladder transitional carcinoma cell line T24 cells.
Li Y, Zhao X, Tang H, Zhong Z, Zhang L, Xu R, Li S, Wang Y. Li Y, et al. Urol Int. 2012;88(1):95-101. doi: 10.1159/000331881. Epub 2011 Oct 25. Urol Int. 2012. PMID: 22041818 - YC-1 targeting of hypoxia-inducible factor-1α reduces RGC-5 cell viability and inhibits cell proliferation.
Tsui L, Fong TH, Wang IJ. Tsui L, et al. Mol Vis. 2012;18:1594-603. Epub 2012 Jun 15. Mol Vis. 2012. PMID: 22736948 Free PMC article. - Effects of YC-1 on hypoxia-inducible factor 1-driven transcription activity, cell proliferative vitality, and apoptosis in hypoxic human pancreatic cancer cells.
Zhao Q, Du J, Gu H, Teng X, Zhang Q, Qin H, Liu N. Zhao Q, et al. Pancreas. 2007 Mar;34(2):242-7. doi: 10.1097/01.mpa.0000250135.95144.b6. Pancreas. 2007. PMID: 17312464 - Advances in antitumor research of HIF-1α inhibitor YC-1 and its derivatives.
Ouyang C, Zhang J, Lei X, Xie Z, Liu X, Li Y, Huang S, Wang Z, Tang G. Ouyang C, et al. Bioorg Chem. 2023 Apr;133:106400. doi: 10.1016/j.bioorg.2023.106400. Epub 2023 Jan 30. Bioorg Chem. 2023. PMID: 36739684 Review. - Synthetic strategy and structure-activity relationship (SAR) studies of 3-(5'-hydroxymethyl-2'-furyl)-1-benzyl indazole (YC-1, Lificiguat): a review.
Yu KH, Hung HY. Yu KH, et al. RSC Adv. 2021 Dec 20;12(1):251-264. doi: 10.1039/d1ra08120a. eCollection 2021 Dec 20. RSC Adv. 2021. PMID: 35424505 Free PMC article. Review.
Cited by
- HIF-1α/Beclin1-Mediated Autophagy Is Involved in Neuroprotection Induced by Hypoxic Preconditioning.
Lu N, Li X, Tan R, An J, Cai Z, Hu X, Wang F, Wang H, Lu C, Lu H. Lu N, et al. J Mol Neurosci. 2018 Oct;66(2):238-250. doi: 10.1007/s12031-018-1162-7. Epub 2018 Sep 10. J Mol Neurosci. 2018. PMID: 30203298 Free PMC article. - YC-1 Antagonizes Wnt/β-Catenin Signaling Through the EBP1 p42 Isoform in Hepatocellular Carcinoma.
Wu JY, Shih YL, Lin SP, Hsieh TY, Lin YW. Wu JY, et al. Cancers (Basel). 2019 May 13;11(5):661. doi: 10.3390/cancers11050661. Cancers (Basel). 2019. PMID: 31086087 Free PMC article. - A simplified protocol to induce hypoxia in a standard incubator: A focus on retinal cells.
Kaur B, Miglioranza Scavuzzi B, F Abcouwer S, N Zacks D. Kaur B, et al. Exp Eye Res. 2023 Nov;236:109653. doi: 10.1016/j.exer.2023.109653. Epub 2023 Oct 2. Exp Eye Res. 2023. PMID: 37793495 Free PMC article. - Loss of Blood-Brain Barrier Integrity in an In Vitro Model Subjected to Intermittent Hypoxia: Is Reversion Possible with a HIF-1α Pathway Inhibitor?
Voirin AC, Chatard M, Briançon-Marjollet A, Pepin JL, Perek N, Roche F. Voirin AC, et al. Int J Mol Sci. 2023 Mar 6;24(5):5062. doi: 10.3390/ijms24055062. Int J Mol Sci. 2023. PMID: 36902491 Free PMC article. - Exploring the Molecular Interactions of 7,8-Dihydroxyflavone and Its Derivatives with TrkB and VEGFR2 Proteins.
Chitranshi N, Gupta V, Kumar S, Graham SL. Chitranshi N, et al. Int J Mol Sci. 2015 Sep 3;16(9):21087-108. doi: 10.3390/ijms160921087. Int J Mol Sci. 2015. PMID: 26404256 Free PMC article.
References
- Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–71. - PubMed
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–82. - PubMed
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70:1469–80. - PubMed
- Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem. 1997;272:11205–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous