The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules - PubMed (original) (raw)
The RNA-binding protein Fus directs translation of localized mRNAs in APC-RNP granules
Kyota Yasuda et al. J Cell Biol. 2013.
Abstract
RNA localization pathways direct numerous mRNAs to distinct subcellular regions and affect many physiological processes. In one such pathway the tumor-suppressor protein adenomatous polyposis coli (APC) targets RNAs to cell protrusions, forming APC-containing ribonucleoprotein complexes (APC-RNPs). Here, we show that APC-RNPs associate with the RNA-binding protein Fus/TLS (fused in sarcoma/translocated in liposarcoma). Fus is not required for APC-RNP localization but is required for efficient translation of associated transcripts. Labeling of newly synthesized proteins revealed that Fus promotes translation preferentially within protrusions. Mutations in Fus cause amyotrophic lateral sclerosis (ALS) and the mutant protein forms inclusions that appear to correspond to stress granules. We show that overexpression or mutation of Fus results in formation of granules, which preferentially recruit APC-RNPs. Remarkably, these granules are not translationally silent. Instead, APC-RNP transcripts are translated within cytoplasmic Fus granules. These results unexpectedly show that translation can occur within stress-like granules. Importantly, they identify a new local function for cytoplasmic Fus with implications for ALS pathology.
Figures
Figure 1.
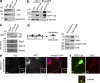
The RNA-binding protein Fus is a component of APC-RNPs at cell protrusions. NIH/3T3 cells untransfected (a and c) or transfected with GFP or GFP-Fus (b) were immunoprecipitated (IP) with the indicated antibodies and analyzed by Western blot (a–c, top panel) or by RT-PCR (c, bottom panels). (d) NIH/3T3 cells were plated on microporous filters, induced to migrate by addition of LPA, and protrusions and cell bodies were isolated and analyzed by Western blot. (e) NIH/3T3 cells were immunostained to detect endogenous Fus and APC. Insets: magnification of the boxed protrusive area. Yellow line in overlay panel: cell outline (f) GFP-Fus–expressing cells immunostained to detect APC. Bars, 10 µm (insets, 3 µm).
Figure 2.
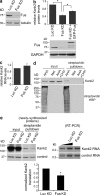
Fus is required for efficient translation of the Kank2-localized mRNA. (a) Western blot of NIH/3T3 cells stably expressing short hairpin RNAs against luciferase (Luc KD) or Fus (Fus KD). (b and c) Kank2 protein (b, top) and RNA levels (c) were detected in Luc KD, Fus KD, or Fus KD cells reexpressing GFP-Fus. n = 5. *, P < 0.05 by Student’s t test. (b, bottom) Western blot showing levels of reexpressed GFP-Fus (d) Cells were labeled with AHA, methionine (Met), or AHA in the presence of cycloheximide for 30 min. AHA-labeled proteins in lysates were biotinylated and purified with streptavidin beads. Kank2 or total biotinylated proteins (streptavidin-HRP) were detected. (e) Newly synthesized proteins from Luc KD or Fus KD cells were isolated as in d (left), and the indicated RNAs were detected by RT-PCR (middle). Graph shows Kank2 protein/RNA after normalization to the corresponding controls (GAPDH/tubulin). *, P = 0.006 by Student’s t test.
Figure 3.
Fus is required for efficient translation in cell protrusions. (a) Diagram showing labeling scheme of cells on microporous filters. (b) Confocal images of protrusions or cell bodies of cells labeled as in panel a with or without cycloheximide. Cells were also immunostained to detect Tubulin. (c) Western blot of Fus levels in control, Fus KD cells, and Fus KD cells reexpressing Fus. (d) Box-plot graph showing values of normalized translation at protrusions of the indicated cell types. *, P < 0.05 by Mann-Whitney test; n.s.: not significant. Bars, 16 µm.
Figure 4.
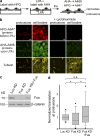
Preferential recruitment of APC-RNPs in cytoplasmic granules formed by an ALS-associated mutant of Fus. (a) NIH/3T3 cells were transfected with GFP-Fus(R521C) (top panels). Middle panels show distribution of coexpressed fluorescently tagged proteins or immunostaining of endogenous proteins. (b) Primary hippocampal neurons were transfected with GFP or GFP-Fus(R521C) and were immunostained at DIV5 to detect APC. (c) Hippocampal sections from a patient with FTLD-Fus (sporadic NIFID) and a control donor were stained for Fus and APC. Nuclei were stained with Draq5. Arrows point to Fus granules. Note that likely both glial cells and neurons are present in these sections. (d) NIH/3T3 cells transfected with GFP- or RFP-Fus(R521C) were analyzed by FISH to detect the Ddr2 or RhoA mRNAs or were cotransfected with DsRed-MS2 and an MS2 reporter RNA carrying the Pkp4 3′UTR (β24bs/Pkp4). Graph shows relative fluorescence intensities of Ddr2 and RhoA mRNAs within the cytoplasm or Fus granules. P-value is by Student’s t test. Bars, 8 µm.
Figure 5.
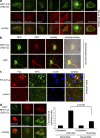
Granules formed by mutant Fus are sites of active translation. (a) Cells expressing RFP-Fus(R521C) were treated or not with cycloheximide (CHX) for 40 min and immunostained with the indicated antibodies (IF Ab). Box-plot graphs show fluorescence values within granules normalized to RFP-Fus signal. *, P < 0.001 by Mann-Whitney test. (b) Cells expressing RFP-Fus(R521C) were labeled for 30 min with AHA (with or without CHX) or with methionine (Met). After fixation, AHA-containing proteins were tagged with Alexa Fluor 488 and visualized. (c) Detection of translation sites in cells expressing RFP-Fus(R521C) using ribopuromycylation. Bars, 8 µm.
Similar articles
- Nuclear transport impairment of amyotrophic lateral sclerosis-linked mutations in FUS/TLS.
Ito D, Seki M, Tsunoda Y, Uchiyama H, Suzuki N. Ito D, et al. Ann Neurol. 2011 Jan;69(1):152-62. doi: 10.1002/ana.22246. Epub 2010 Dec 8. Ann Neurol. 2011. PMID: 21280085 - Aggregation of ALS-linked FUS mutant sequesters RNA binding proteins and impairs RNA granules formation.
Takanashi K, Yamaguchi A. Takanashi K, et al. Biochem Biophys Res Commun. 2014 Sep 26;452(3):600-7. doi: 10.1016/j.bbrc.2014.08.115. Epub 2014 Aug 28. Biochem Biophys Res Commun. 2014. PMID: 25173930 - ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay.
Kamelgarn M, Chen J, Kuang L, Jin H, Kasarskis EJ, Zhu H. Kamelgarn M, et al. Proc Natl Acad Sci U S A. 2018 Dec 18;115(51):E11904-E11913. doi: 10.1073/pnas.1810413115. Epub 2018 Nov 19. Proc Natl Acad Sci U S A. 2018. PMID: 30455313 Free PMC article. - Stress granules in neurodegeneration--lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma.
Bentmann E, Haass C, Dormann D. Bentmann E, et al. FEBS J. 2013 Sep;280(18):4348-70. doi: 10.1111/febs.12287. Epub 2013 May 9. FEBS J. 2013. PMID: 23587065 Review. - Altered mRNP granule dynamics in FTLD pathogenesis.
Bowden HA, Dormann D. Bowden HA, et al. J Neurochem. 2016 Aug;138 Suppl 1:112-33. doi: 10.1111/jnc.13601. Epub 2016 Jun 15. J Neurochem. 2016. PMID: 26938019 Review.
Cited by
- Local mRNA translation and cytoskeletal reorganization: Mechanisms that tune neuronal responses.
Triantopoulou N, Vidaki M. Triantopoulou N, et al. Front Mol Neurosci. 2022 Aug 1;15:949096. doi: 10.3389/fnmol.2022.949096. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35979146 Free PMC article. Review. - ALS-linked cytoplasmic FUS assemblies are compositionally different from physiological stress granules and sequester hnRNPA3, a novel modifier of FUS toxicity.
An H, Litscher G, Watanabe N, Wei W, Hashimoto T, Iwatsubo T, Buchman VL, Shelkovnikova TA. An H, et al. Neurobiol Dis. 2022 Jan;162:105585. doi: 10.1016/j.nbd.2021.105585. Epub 2021 Dec 14. Neurobiol Dis. 2022. PMID: 34915152 Free PMC article. - Post-transcriptional control of fungal cell wall synthesis.
Hall RA, Wallace EWJ. Hall RA, et al. Cell Surf. 2022 Jan 12;8:100074. doi: 10.1016/j.tcsw.2022.100074. eCollection 2022 Dec. Cell Surf. 2022. PMID: 35097244 Free PMC article. - Stress Granules as Causes and Consequences of Translation Suppression.
Baymiller M, Moon SL. Baymiller M, et al. Antioxid Redox Signal. 2023 Aug;39(4-6):390-409. doi: 10.1089/ars.2022.0164. Epub 2023 Jun 28. Antioxid Redox Signal. 2023. PMID: 37183403 Free PMC article. Review. - ALS-linked FUS mutations confer loss and gain of function in the nucleus by promoting excessive formation of dysfunctional paraspeckles.
An H, Skelt L, Notaro A, Highley JR, Fox AH, La Bella V, Buchman VL, Shelkovnikova TA. An H, et al. Acta Neuropathol Commun. 2019 Jan 14;7(1):7. doi: 10.1186/s40478-019-0658-x. Acta Neuropathol Commun. 2019. PMID: 30642400 Free PMC article.
References
- Andersson M.K., Ståhlberg A., Arvidsson Y., Olofsson A., Semb H., Stenman G., Nilsson O., Aman P. 2008. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol. 9:37 10.1186/1471-2121-9-37 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous