Survey of innate immune responses to Burkholderia pseudomallei in human blood identifies a central role for lipopolysaccharide - PubMed (original) (raw)
. 2013 Nov 26;8(11):e81617.
doi: 10.1371/journal.pone.0081617. eCollection 2013.
Sarunporn Tandhavanant, Nicolle D Myers, Sudeshna Seal, Arkhom Arayawichanont, Aroonsri Kliangsa-Ad, Lauren E Hittle, Robert K Ernst, Mary J Emond, Mark M Wurfel, Nicholas P J Day, Sharon J Peacock, T Eoin West
Affiliations
- PMID: 24303060
- PMCID: PMC3841221
- DOI: 10.1371/journal.pone.0081617
Survey of innate immune responses to Burkholderia pseudomallei in human blood identifies a central role for lipopolysaccharide
Narisara Chantratita et al. PLoS One. 2013.
Abstract
B. pseudomallei is a gram-negative bacterium that causes the tropical infection melioidosis. In northeast Thailand, mortality from melioidosis approaches 40%. As exemplified by the lipopolysaccharide-Toll-like receptor 4 interaction, innate immune responses to invading bacteria are precipitated by activation of host pathogen recognition receptors by pathogen associated molecular patterns. Human melioidosis is characterized by up-regulation of pathogen recognition receptors and pro-inflammatory cytokine release. In contrast to many gram-negative pathogens, however, the lipopolysaccharide of B. pseudomallei is considered only weakly inflammatory. We conducted a study in 300 healthy Thai subjects to investigate the ex vivo human blood response to various bacterial pathogen associated molecular patterns, including lipopolysaccharide from several bacteria, and to two heat-killed B. pseudomallei isolates. We measured cytokine levels after stimulation of fresh whole blood with a panel of stimuli. We found that age, sex, and white blood cell count modulate the innate immune response to B. pseudomallei. We further observed that, in comparison to other stimuli, the innate immune response to B. pseudomallei is most highly correlated with the response to lipopolysaccharide. The magnitude of cytokine responses induced by B. pseudomallei lipopolysaccharide was significantly greater than those induced by lipopolysaccharide from Escherichia coli and comparable to many responses induced by lipopolysaccharide from Salmonella minnesota despite lower amounts of lipid A in the B. pseudomallei lipopolysaccharide preparation. In human monocytes stimulated with B. pseudomallei, addition of polymyxin B or a TLR4/MD-2 neutralizing antibody inhibited the majority of TNF-α production. Challenging existing views, our data indicate that the innate immune response to B. pseudomallei in human blood is largely driven by lipopolysaccharide, and that the response to B. pseudomallei lipopolysaccharide in blood is greater than the response to other lipopolysaccharide expressing isolates. Our findings suggest that B. pseudomallei lipopolysaccharide may play a central role in stimulating the host response in melioidosis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
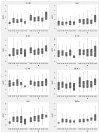
Figure 1. WBC-normalized plasma cytokine concentrations induced by stimulation of whole blood from 300 healthy subjects at 37°C for six hours with medium alone, E. coli O111:B4 LPS 10 ng/ml (as a positive control), heat-killed B. pseudomallei 1026b 2.5 × 106 CFU/ml, or heat-killed B. pseudomallei K96243 2.5 × 106 CFU/ml.
Boxes show the median and interquartile range; whiskers show upper and lower adjacent values; outside values are not shown for clarity.
Figure 2. WBC-normalized plasma cytokine concentrations induced by stimulation of whole blood from 300 healthy subjects at 37°C for six hours with heat-killed B. pseudomallei 1026b 2.5 × 106 CFU/ml, stratified by age and sex.
Boxes show the median and interquartile range; whiskers show upper and lower adjacent values; outside values are not shown for clarity.
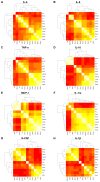
Figure 3. Heat map of correlation between cytokine concentration induced by stimulation of whole blood with a panel of purified innate immune ligands and heat-killed bacteria (as specified in the methods) with dendrogram of hierarchichal clustering results for the correlation matrix.
Lowest correlation is red and highest correlation is white.
Figure 4. Blockade of the LPS-TLR4 axis markedly impairs TNF-α production induced by B. pseudomallei in human monocytes.
Human monocytes (50,000/well) were stimulated with B. pseudomallei K96243 LPS 1 ng/ml (LPS) or heat-killed B. pseudomallei K96243 (bacteria:monocyte ratio of 1:1) (BP), with or without polymyxin B 100 μg/ml (PB) or a TLR4/MD2 neutralizing antibody 20 μg/ml (Ab) or isotype control 20 μg/ml (Iso). TNF-α was assayed in duplicate cell supernatants after 4 hours. Given substantial inter-individual variation in cytokine release, all TNF-α values for each individual were normalized to LPS-induced levels from the same subject. Relative TNF- α units for each individual (N=4) and mean values are displayed. Statistically significant differences were determined by ANOVA and a Bonferroni post-test. For clarity, only differences between each agonist alone (LPS or BP) and each agonist with polymyxin B or an antibody are shown. ***, p≤0.001. NS, p>0.05.
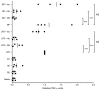
Figure 5. Plasma cytokine concentrations induced by stimulation of whole blood with B. pseudomallei K96243 LPS 10 ng/ml (BPlps), E. coli O111:B4 LPS 10 ng/ml (EClps), or S. minnesota Re595 LPS 10 ng/ml (SMlps).
Boxes show the median and interquartile range; whiskers show upper and lower adjacent values; outside values are not shown for clarity. Statistical comparisons are made by ANOVA with a Bonferroni post test on log10 transformed data. *, p≤0.05; **, p≤0.01; ***, p≤0.001.
Similar articles
- Activation of Toll-like receptors by Burkholderia pseudomallei.
West TE, Ernst RK, Jansson-Hutson MJ, Skerrett SJ. West TE, et al. BMC Immunol. 2008 Aug 8;9:46. doi: 10.1186/1471-2172-9-46. BMC Immunol. 2008. PMID: 18691413 Free PMC article. - Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (Melioidosis).
Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, Chierakul W, Leendertse M, Florquin S, de Vos AF, White N, Dondorp AM, Day NP, Peacock SJ, van der Poll T. Wiersinga WJ, et al. PLoS Med. 2007 Jul 31;4(7):e248. doi: 10.1371/journal.pmed.0040248. PLoS Med. 2007. PMID: 17676990 Free PMC article. - Comprehensive analysis of clinical Burkholderia pseudomallei isolates demonstrates conservation of unique lipid A structure and TLR4-dependent innate immune activation.
Sengyee S, Yoon SH, Paksanont S, Yimthin T, Wuthiekanun V, Limmathurotsakul D, West TE, Ernst RK, Chantratita N. Sengyee S, et al. PLoS Negl Trop Dis. 2018 Feb 23;12(2):e0006287. doi: 10.1371/journal.pntd.0006287. eCollection 2018 Feb. PLoS Negl Trop Dis. 2018. PMID: 29474381 Free PMC article. - Immunity to Burkholderia pseudomallei.
Wiersinga WJ, van der Poll T. Wiersinga WJ, et al. Curr Opin Infect Dis. 2009 Apr;22(2):102-8. doi: 10.1097/QCO.0b013e328322e727. Curr Opin Infect Dis. 2009. PMID: 19276877 Review. - Immune response to Burkholderia pseudomallei.
Panomket P. Panomket P. J Med Assoc Thai. 2011 Nov;94(11):1410-7. J Med Assoc Thai. 2011. PMID: 22256484 Review.
Cited by
- Dysfunctional host cellular immune responses are associated with mortality in melioidosis.
Wright SW, Ekchariyawat P, Sengyee S, Phunpang R, Dulsuk A, Saiprom N, Thiansukhon E, Pattanapanyasat K, Korbsrisate S, West TE, Chantratita N. Wright SW, et al. Emerg Microbes Infect. 2024 Dec;13(1):2380822. doi: 10.1080/22221751.2024.2380822. Epub 2024 Jul 31. Emerg Microbes Infect. 2024. PMID: 39008280 Free PMC article. - NLRC4 and TLR5 each contribute to host defense in respiratory melioidosis.
West TE, Myers ND, Chantratita N, Chierakul W, Limmathurotsakul D, Wuthiekanun V, Miao EA, Hajjar AM, Peacock SJ, Liggitt HD, Skerrett SJ. West TE, et al. PLoS Negl Trop Dis. 2014 Sep 18;8(9):e3178. doi: 10.1371/journal.pntd.0003178. eCollection 2014 Sep. PLoS Negl Trop Dis. 2014. PMID: 25232720 Free PMC article. - Immune response to recombinant Burkholderia pseudomallei FliC.
Koosakulnirand S, Phokrai P, Jenjaroen K, Roberts RA, Utaisincharoen P, Dunachie SJ, Brett PJ, Burtnick MN, Chantratita N. Koosakulnirand S, et al. PLoS One. 2018 Jun 14;13(6):e0198906. doi: 10.1371/journal.pone.0198906. eCollection 2018. PLoS One. 2018. PMID: 29902230 Free PMC article. - A reverse-phase protein microarray-based screen identifies host signaling dynamics upon Burkholderia spp. infection.
Chiang CY, Uzoma I, Lane DJ, Memišević V, Alem F, Yao K, Kota KP, Bavari S, Wallqvist A, Hakami RM, Panchal RG. Chiang CY, et al. Front Microbiol. 2015 Jul 27;6:683. doi: 10.3389/fmicb.2015.00683. eCollection 2015. Front Microbiol. 2015. PMID: 26284031 Free PMC article. - Implications of environmental and pathogen-specific determinants on clinical presentations and disease outcome in melioidosis patients.
Shaw T, Tellapragada C, Kamath A, Kalwaje Eshwara V, Mukhopadhyay C. Shaw T, et al. PLoS Negl Trop Dis. 2019 May 15;13(5):e0007312. doi: 10.1371/journal.pntd.0007312. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31091290 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- U54 AI057141/AI/NIAID NIH HHS/United States
- K08 HL094759/HL/NHLBI NIH HHS/United States
- R01 HL113382/HL/NHLBI NIH HHS/United States
- 087769/Z/08/Z/WT_/Wellcome Trust/United Kingdom
- R01HL113382/HL/NHLBI NIH HHS/United States
- U54AI057141/AI/NIAID NIH HHS/United States
- R01HL089807/HL/NHLBI NIH HHS/United States
- Wellcome Trust/United Kingdom
- R01 HL089807/HL/NHLBI NIH HHS/United States
- K08HL094759/HL/NHLBI NIH HHS/United States
- 089275/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials