ALS-associated TDP-43 induces endoplasmic reticulum stress, which drives cytoplasmic TDP-43 accumulation and stress granule formation - PubMed (original) (raw)
ALS-associated TDP-43 induces endoplasmic reticulum stress, which drives cytoplasmic TDP-43 accumulation and stress granule formation
Adam K Walker et al. PLoS One. 2013.
Abstract
In amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration, TAR DNA binding protein 43 (TDP-43) accumulates in the cytoplasm of affected neurons and glia, where it associates with stress granules (SGs) and forms large inclusions. SGs form in response to cellular stress, including endoplasmic reticulum (ER) stress, which is induced in both familial and sporadic forms of ALS. Here we demonstrate that pharmacological induction of ER stress causes TDP-43 to accumulate in the cytoplasm, where TDP-43 also associates with SGs. Furthermore, treatment with salubrinal, an inhibitor of dephosphorylation of eukaryotic initiation factor 2-α, a key modulator of ER stress, potentiates ER stress-mediated SG formation. Inclusions of C-terminal fragment TDP-43, reminiscent of disease-pathology, form in close association with ER and Golgi compartments, further indicating the involvement of ER dysfunction in TDP-43-associated disease. Consistent with this notion, over-expression of ALS-linked mutant TDP-43, and to a lesser extent wildtype TDP-43, triggers several ER stress pathways in neuroblastoma cells. Similarly, we found an interaction between the ER chaperone protein disulphide isomerase and TDP-43 in transfected cell lysates and in the spinal cords of mutant A315T TDP-43 transgenic mice. This study provides evidence for ER stress as a pathogenic pathway in TDP-43-mediated disease.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
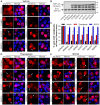
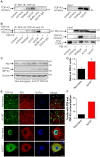
Figure 1. ER stress and proteasome inhibition cause redistribution of wildtype and ALS-linked mutant TDP-43.
(A) Neuro2a cells were transfected with the construct indicated to the left of each series of panels (mCherry alone, wildtype TDP-43-mCherry, or mutant D169G, G294A, A315T, Q331K, M337V or N390D TDP-43-mCherry), and fixed at 48 h post-transfection. mCherry fluorescence is shown in the left panels, and Hoechst nuclei stain is shown in the merged right panels. Note the largely nuclear distribution of wildtype and mutant TDP-43-mCherry, but with low levels of non-nuclear mCherry fluorescence in some cells, indicated by arrows. (B) Immunoblot of cell lysates expressing mCherry alone or wildtype or mutant TDP-43-mCherry showing equal expression levels of all proteins, and levels of endogenous (End.) TDP-43. Approximate molecular weight markers are shown on the right. (C) Quantification of the effect of thapsigargin or MG132 treatment on the percentage of Neuro2a cells with cytoplasmic mCherry fluorescence. Results are expressed as mean ± SEM, n = 3, *p<0.05 versus respective vehicle treated controls by two-way ANOVA with Bonferroni's post-test. (D) Neuro2a cells were transfected as in A and treated with 100 nM thapsigargin for 24 h prior to fixation. (E) Neuro2a cells were transfected as in A and treated with 10 µM MG132 for 24 h prior to fixation. Note the increased non-nuclear distribution of wildtype and mutant TDP-43-mCherry with both thapsigargin and MG132 treatment, indicated by arrows. All scale bars represent 10 µm.
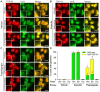
Figure 2. Formation of ER stress-induced TDP-43-positive stress granules is enhanced by salubrinal pre-treatment.
HeLa cells were pre-treated for 2 h with either vehicle control, 50 µM salubrinal or 50 µg/mL cycloheximide and then stressed for an additional 1 h by treatment with vehicle control (A), 0.5 mM arsenite (B), or 10 µM thapsigargin (C) in the presence of the respective pre-treatment conditions. Cells were processed for immunocytochemistry using antibodies against TDP-43 (left panels) and HuR (middle panels). Merged images are shown on the right. Boxed regions in the main panels are shown magnified in the bottom left of each panel. (D) Quantification of the effect of aresenite or thapsigargin in the presence of salubrinal or cycloheximide on the formation of HuR-positive or HuR/TDP-43-positive cytoplasmic SGs. Results are expressed as mean ± SEM, n = 3, *p<0.05 versus respective vehicle-treated controls, #p<0.05 versus vehicle pre-treated/thapsigargin treated control, by two-way ANOVA followed by Bonferroni's post-test. All scale bars represent 20 µm.
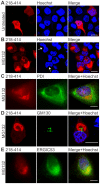
Figure 3. C-terminal 218–414 TDP-43 inclusions form in association with the Golgi and ER.
(A, B) Expression of wildtype 218–414 TDP-43-mCherry in Neuro2a cells, with mCherry fluorescence (left images), Hoechst nuclei stain (middle images), and merged images (right). Panel A shows untreated cells, and panel B shows cells treated with 10 µM MG132 for 24 h. Arrows indicate nuclei showing distorted morphology, due to the presence of inclusions. Note that the mCherry fluorescence image shown in A was captured using increased gain and offset settings compared to that shown in B in order to allow detection. (C) MG132-treated HeLa cells expressing 218–414 TDP-43-mCherry (red, left image), with immunocytochemistry for PDI (green, middle image). (D) Neuro2a cells were transfected and treated as in B and processed for immunocytochemistry against GM130 (green, middle image). In MG132-treated HeLa cells expressing 218-414 TDP-43-mCherry (red, left image), immunocytochemistry is shown for (E) ERGIC53 (green, middle image). Merged images shown with Hoechst nuclei stain are on the right. All scale bars represent 10 µm.
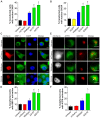
Figure 4. Wildtype and ALS-linked mutant TDP-43 proteins induce ER stress.
(A) Increase in nuclear CHOP immunoreactivity in Neuro2a cells expressing wildtype and ALS mutant forms of TDP-43 linked with mCherry. (B) Increase in nuclear CHOP immunoreactivity in Neuro2a cells expressing wildtype and ALS mutant forms of TDP-43 linked with EGFP. (C) XBP-1 is activated in mutant TDP-43 Q331K cells, indicating induction of the IRE1 pathway of ER stress. Cells expressing TDP-43 mCherry (first column, red) are shown with XBP-1 (second column, green) and DAPI staining (third column, blue). Merge (fourth column) indicates overlays of the fluorescent images of XBP-1 and DAPI. Arrow indicates cells with increased nuclear XBP-1. Scale bars represent 5 µm. (D) Quantification of transfected cells with activation of XBP-1, as determined by an increase in nuclear XBP-1 immunoreactivity. (E) ATF6 translocates to the Golgi in mutant Q331K cells, indicating activation of the ATF6 pathway of ER stress. Neuro2a cells were co-transfected with mCherry constructs (first column, white) and ATF6-GFP (second column, green). Cells were fixed and immunostained for Golgi marker GM130 (third column, red). Merge (fourth column) indicates overlays of the fluorescent images of ATF6 and GM130. Arrow indicates co-localisation of ATF6 in the Golgi apparatus. Scale bars represent 10 µm. (F) Quantification of transfected cells with activation of ATF6, as determined by co-localisation of ATF6 with GM130. Data are represented as mean ± SEM; *p<0.05 versus mCherry or EGFP controls and #p<0.05 versus respective wildtype TDP-43 proteins, by one-way ANOVA with Tukey's post hoc test.
Figure 5. PDI co-precipitates with TDP-43 and is increased in mutant TDP-43 transgenic mouse spinal cords.
(A) Co-immunoprecipitation of wildtype and mutant Q331K TDP-43-mCherry from Neuro2a cell lysates using an anti-PDI antibody followed by TDP-43 immunoblot. Control immunoprecipitations were performed using untransfected (Untrans) or mCherry expressing cell lysates, buffer only with coprecipitating antibodies or TDP-43 Q331K cell lysates using an irrelevant, isotype-matched control antibody (IgG). Input control (2%) shows expression of mCherry-TDP43 in wildtype and Q331K cell lysates. (B) Co-immunoprecipitation of PDI-V5 from wildype and mutant A315T and Q331K TDP-43-mCherry from HEK293T cell lysates using an anti-RFP antibody to precipitate mCherry followed by TDP-43 and V5 immunoblot. Control immunoprecipitations were performed using Untrans cell lysates or mCherry and PDI-V5 expressing cell lysates, buffer only with coprecipitating antibody or Q331K TDP-43 and PDI-V5 co-transfected cell lysates using an irrelevant, isotype-matched control antibody (IgG). Input control shows expression of PDI-V5, TDP-43-mCherry and endogenous (End.) TDP-43. Approximate molecular weight markers are shown on the right, and representative images are shown. (C) Proteins were extracted from spinal cords of three mutant A315T TDP-43 transgenic mice and three litter-matched non-transgenic controls, and immunoblotting was performed for PDI, FLAG-TDP-43 (detected using antibody against FLAG), and β-actin. (D) Quantification of PDI levels from immunoblots normalised to β-actin by densitometry. Data represent mean normalised values from three independent analyses and are shown as mean ± SEM. *p<0.05 versus non-transgenic controls by unpaired two-tailed t-test. (E) Immunohistochemistry for TDP-43 and PDI in non-transgenic and A315T mutant TDP-43 mouse spinal cords. Cells were immunostained for TDP-43 (first column, green), PDI (second column, red), and were stained using To-Pro to identify nuclei (third column, blue). Merged images (fourth column) are also shown. Increased co-localisation of PDI with TDP-43 is seen in A315T animals compared to non-transgenic controls (Non-trans). All scale bars represent 10 µm. (F) Quantification of the percentage of motor neurons in which PDI and TDP-43 were co-localised. A total of 60 cells per group were counted, and data represent mean values from two mice per group.
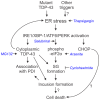
Figure 6. A model for the involvement of ER stress in TDP-43 proteinopathies.
ER stress activation causes accumulation of cytoplasmic TDP-43, and induction of TDP-43-positive SGs, which could lead to inclusion formation and neuron death in disease. ER stress may also lead to cell death via apoptotic signalling involving the transcription factor CHOP, independent of SGs and inclusion formation. Additionally, TDP-43 shows increased association with the chaperone PDI in disease. Pharmacological agents used in this study are shown italicised in blue.
Similar articles
- Rab1-dependent ER-Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS.
Soo KY, Halloran M, Sundaramoorthy V, Parakh S, Toth RP, Southam KA, McLean CA, Lock P, King A, Farg MA, Atkin JD. Soo KY, et al. Acta Neuropathol. 2015 Nov;130(5):679-97. doi: 10.1007/s00401-015-1468-2. Epub 2015 Aug 23. Acta Neuropathol. 2015. PMID: 26298469 - ERp57 is protective against mutant SOD1-induced cellular pathology in amyotrophic lateral sclerosis.
Parakh S, Jagaraj CJ, Vidal M, Ragagnin AMG, Perri ER, Konopka A, Toth RP, Galper J, Blair IP, Thomas CJ, Walker AK, Yang S, Spencer DM, Atkin JD. Parakh S, et al. Hum Mol Genet. 2018 Apr 15;27(8):1311-1331. doi: 10.1093/hmg/ddy041. Hum Mol Genet. 2018. PMID: 29409023 - Protein disulphide isomerase (PDI) is protective against amyotrophic lateral sclerosis (ALS)-related mutant Fused in Sarcoma (FUS) in in vitro models.
Parakh S, Perri ER, Vidal M, Sultana J, Shadfar S, Mehta P, Konopka A, Thomas CJ, Spencer DM, Atkin JD. Parakh S, et al. Sci Rep. 2021 Sep 2;11(1):17557. doi: 10.1038/s41598-021-96181-2. Sci Rep. 2021. PMID: 34475430 Free PMC article. - Conjoint pathologic cascades mediated by ALS/FTLD-U linked RNA-binding proteins TDP-43 and FUS.
Ito D, Suzuki N. Ito D, et al. Neurology. 2011 Oct 25;77(17):1636-43. doi: 10.1212/WNL.0b013e3182343365. Epub 2011 Sep 28. Neurology. 2011. PMID: 21956718 Free PMC article. Review. - "STRESSED OUT": The role of FUS and TDP-43 in amyotrophic lateral sclerosis.
Aksoy YA, Deng W, Stoddart J, Chung R, Guillemin G, Cole NJ, Neely GG, Hesselson D. Aksoy YA, et al. Int J Biochem Cell Biol. 2020 Sep;126:105821. doi: 10.1016/j.biocel.2020.105821. Epub 2020 Aug 3. Int J Biochem Cell Biol. 2020. PMID: 32758633 Review.
Cited by
- Impaired NHEJ repair in amyotrophic lateral sclerosis is associated with TDP-43 mutations.
Konopka A, Whelan DR, Jamali MS, Perri E, Shahheydari H, Toth RP, Parakh S, Robinson T, Cheong A, Mehta P, Vidal M, Ragagnin AMG, Khizhnyak I, Jagaraj CJ, Galper J, Grima N, Deva A, Shadfar S, Nicholson GA, Yang S, Cutts SM, Horejsi Z, Bell TDM, Walker AK, Blair IP, Atkin JD. Konopka A, et al. Mol Neurodegener. 2020 Sep 9;15(1):51. doi: 10.1186/s13024-020-00386-4. Mol Neurodegener. 2020. PMID: 32907630 Free PMC article. - The Redox Activity of Protein Disulfide Isomerase Inhibits ALS Phenotypes in Cellular and Zebrafish Models.
Parakh S, Shadfar S, Perri ER, Ragagnin AMG, Piattoni CV, Fogolín MB, Yuan KC, Shahheydari H, Don EK, Thomas CJ, Hong Y, Comini MA, Laird AS, Spencer DM, Atkin JD. Parakh S, et al. iScience. 2020 May 22;23(5):101097. doi: 10.1016/j.isci.2020.101097. Epub 2020 Apr 25. iScience. 2020. PMID: 32446203 Free PMC article. - Capturing intracellular Ca2+ dynamics in computational models of neurodegenerative diseases.
Anwar H. Anwar H. Drug Discov Today Dis Models. 2016 Spring;19:37-42. doi: 10.1016/j.ddmod.2017.02.005. Epub 2017 Mar 18. Drug Discov Today Dis Models. 2016. PMID: 28983320 Free PMC article. - CDNF rescues motor neurons in models of amyotrophic lateral sclerosis by targeting endoplasmic reticulum stress.
De Lorenzo F, Lüningschrör P, Nam J, Beckett L, Pilotto F, Galli E, Lindholm P, Rüdt von Collenberg C, Mungwa ST, Jablonka S, Kauder J, Thau-Habermann N, Petri S, Lindholm D, Saxena S, Sendtner M, Saarma M, Voutilainen MH. De Lorenzo F, et al. Brain. 2023 Sep 1;146(9):3783-3799. doi: 10.1093/brain/awad087. Brain. 2023. PMID: 36928391 Free PMC article. - Prion-Like Propagation of Protein Misfolding and Aggregation in Amyotrophic Lateral Sclerosis.
McAlary L, Plotkin SS, Yerbury JJ, Cashman NR. McAlary L, et al. Front Mol Neurosci. 2019 Nov 1;12:262. doi: 10.3389/fnmol.2019.00262. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31736708 Free PMC article.
References
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, et al. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133. - PubMed
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, et al. (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous