Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy - PubMed (original) (raw)
Review
Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy
J M Brown. Br J Radiol. 2014 Mar.
Abstract
Tumours have two main ways to develop a vasculature: by angiogenesis, the sprouting of endothelial cells from nearby blood vessels, and vasculogenesis, the formation of blood vessels from circulating cells. Because tumour irradiation abrogates local angiogenesis, the tumour must rely on the vasculogenesis pathway for regrowth after irradiation. Tumour irradiation produces a marked influx of CD11b(+) myeloid cells (macrophages) into the tumours, and these are crucial to the formation of blood vessels in the tumours after irradiation and for the recurrence of the tumours. This process is driven by increased tumour hypoxia, which increases levels of HIF-1 (hypoxia-inducible factor 1), which in turn upregulates SDF-1 (stromal cell-derived factor 1 or CXCL12), the main driver of the vasculogenesis pathway. Inhibition of HIF-1 or of its downstream target SDF-1 prevents the radiation-induced influx of the CD11b(+) myeloid cells and delays or prevents the tumours from recurring following irradiation. Others and we have shown that with a variety of tumours in both mice and rats, the inhibition of the SDF-1/CXCR4 pathway delays or prevents the recurrence of implanted or autochthonous tumours following irradiation or following treatment with vascular disrupting agents or some chemotherapeutic drugs such as paclitaxel. In addition to the recruited macrophages, endothelial progenitor cells (EPCs) are also recruited to the irradiated tumours, a process also driven by SDF-1. Together, the recruited proangiogenic macrophages and the EPCs reform the tumour vasculature and allow the tumour to regrow following irradiation. This is a new paradigm with major implications for the treatment of solid tumours by radiotherapy.
Figures
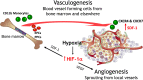
Figure 1.
Cartoon of the two main ways for tumours to develop a functioning vasculature. Also shown are the two principal cytokines governing these pathways: vascular endothelial growth factor (VEGF) for angiogenesis and stromal cell-derived factor 1 (SDF-1; CXCL12) for vasculogenesis. Tumour hypoxia through its upregulation of levels of the transcription factor HIF-1 (hypoxia inducible factor 1) is the main driver of both processes. EPC, endothelial progenitor cell; PPC, pericyte progenitor cell.
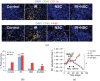
Figure 2.
Myeloid cells (macrophages) are recruited into irradiated tumours, and inhibition of HIF (hypoxia inducible factor) abrogates this influx and prevents tumour recurrence. (a) immunohistochemistry (IHC) staining for leukocyte (CD45) and monocyte (CD11b, top row) or macrophage (F4/80, bottom row) infiltration into i.c. (intracranial) control or 12-Gy treated tumours. Tumours were harvested on the day of irradiation for controls and 17 days after irradiation in treatment groups. Scale bar: 50 μm. (b) Quantification of CD11b+ and F4/80+ cell influx in tumours. Error bars indicate standard deviation. **p < 0.01, ***p < 0.001 versus control. (c) Growth curves (by bioluminescence imaging, BLI) of U251 tumours growing in the brains of nude mice and given 15 Gy with or without treatment of the mice with the HIF inhibitor NSC 134754 for 21 days started immediately after irradiation. *p < 0.05. Adapted from Kioi et al with permission. DAPI, 4′,6-diamidino-2-phenylindole; IR, irradiation.
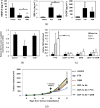
Figure 3.
Therapeutic effect of blocking the interaction of stromal cell-derived factor 1 (SDF-1) with CXCR4 after whole-brain irradiation. (a) Growth curves of i.c. (intracranial) U251 early tumour model after 5 daily doses of 2 Gy starting on Day 11 after transplantation. *p < 0.05. (b) Growth curves of i.c. U251 advanced tumour model after a single dose of irradiation (15 Gy on Day 22 after transplantation), treated with AMD3100 (21-day infusion). (c) As in (b) but with neutralizing anti-CXCR4 Abs instead of AMD3100, *p < 0.05). (d) Growth curves of U251 i.c. tumour after 15-Gy irradiation, treated with the anti-vascular endothelial growth factor-R antibody DC101. Arrowheads indicate the treatment of DC101 (started immediately after irradiation and maintained for 21 days). Adapted from Kioi et al with permission.
Figure 4.
Circulating levels of growth factors after paclitaxel (PTX) or gemcitabine (GEM) treatment and effect of anti-SDF-1 antibody (Ab) on endothelial progenitor cells (EPCs) and tumour growth. (a) Non-tumour-bearing C57BL/6 mice (n = 4 mice per group) were treated with PTX or GEM. 4 h later, mice were bled by cardiac puncture and plasma was collected to measure vascular endothelial growth factor (VEGF)-A, SDF-1α and granulocyte-colony stimulating factor levels by enzyme-linked immunosorbent assay. (b) Analysis of SDF-1α content stored in isolated circulating platelets from C57BL/6 mice 4 h after treatment with PTX or GEM at the maximum tolerated dose (MTD). (c) Non-tumour-bearing C57BL/6 mice (n = 5 mice per group) were treated with SDF-1α neutralizing antibodies. 24 h later, mice were treated with PTX or GEM. After 4 and 24 h, mice were bled from the retro orbital sinus for evaluation of viable endothelial progenitor cells by flow cytometry. (d) C57BL/6 mice bearing Lewis lung carcinoma (LLC) tumours (500 mm3) were treated with polyclonal SDF-1α neutralizing antibodies in combination with either PTX or GEM. Control mice received non-specific antiserum treatment. Data are expressed as mean ± standard deviation. 0.05 > *p > 0.01; **p < 0.01. From Shaked et al with permission. G-CSF, granulocyte colony-stimulating factor.
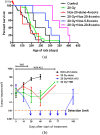
Figure 5.
Stromal cell-derived factor 1 (SDF-1) inhibition after irradiation prolongs the survival of the brain tumour-bearing rats and produces tumour remission. Rats born to mothers treated with a single injection of the carcinogen ENU on Day 18 of gestation were sham irradiated or given a dose of 20 Gy to the whole brain with shielding of the buccal cavity. (a) Rats receiving NOX-A12 were injected subcutaneously every 2 days with either 5 or 20 mg kg−1 starting soon after irradiation and continued for either 4 or 8 weeks. (b) Addition of the SDF-1 inhibitor NOX-A12 following irradiation of the ENU-induced brain tumours produces complete responses by MRI. In utero ENU-treated rats were imaged by MR starting on Day 130 of age and then repeated every 2 weeks until death. Adapted from Liu et al with permission. TMZ, temozolomide.
Similar articles
- Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice.
Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Kioi M, et al. J Clin Invest. 2010 Mar;120(3):694-705. doi: 10.1172/JCI40283. Epub 2010 Feb 22. J Clin Invest. 2010. PMID: 20179352 Free PMC article. - Inhibiting vasculogenesis after radiation: a new paradigm to improve local control by radiotherapy.
Martin BJ. Martin BJ. Semin Radiat Oncol. 2013 Oct;23(4):281-7. doi: 10.1016/j.semradonc.2013.05.002. Semin Radiat Oncol. 2013. PMID: 24012342 Free PMC article. Review. - Radiation Damage to Tumor Vasculature Initiates a Program That Promotes Tumor Recurrences.
Brown JM. Brown JM. Int J Radiat Oncol Biol Phys. 2020 Nov 1;108(3):734-744. doi: 10.1016/j.ijrobp.2020.05.028. Epub 2020 May 28. Int J Radiat Oncol Biol Phys. 2020. PMID: 32473180 Review. - Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas.
Tseng D, Vasquez-Medrano DA, Brown JM. Tseng D, et al. Br J Cancer. 2011 Jun 7;104(12):1805-9. doi: 10.1038/bjc.2011.169. Epub 2011 May 17. Br J Cancer. 2011. PMID: 21587260 Free PMC article. Review. - Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats.
Liu SC, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, Merchant M, Zboralski D, Zöllner S, Kruschinski A, Klussmann S, Recht L, Brown JM. Liu SC, et al. Neuro Oncol. 2014 Jan;16(1):21-8. doi: 10.1093/neuonc/not149. Epub 2013 Dec 10. Neuro Oncol. 2014. PMID: 24335554 Free PMC article.
Cited by
- The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence.
Barker HE, Paget JT, Khan AA, Harrington KJ. Barker HE, et al. Nat Rev Cancer. 2015 Jul;15(7):409-25. doi: 10.1038/nrc3958. Nat Rev Cancer. 2015. PMID: 26105538 Free PMC article. Review. - Combined Radiochemotherapy: Metalloproteinases Revisited.
Waller V, Pruschy M. Waller V, et al. Front Oncol. 2021 May 13;11:676583. doi: 10.3389/fonc.2021.676583. eCollection 2021. Front Oncol. 2021. PMID: 34055644 Free PMC article. Review. - A Bloody Conspiracy- Blood Vessels and Immune Cells in the Tumor Microenvironment.
Terrassoux L, Claux H, Bacari S, Meignan S, Furlan A. Terrassoux L, et al. Cancers (Basel). 2022 Sep 21;14(19):4581. doi: 10.3390/cancers14194581. Cancers (Basel). 2022. PMID: 36230504 Free PMC article. Review. - Radiation-Induced Transformation of Immunoregulatory Networks in the Tumor Stroma.
Martinez-Zubiaurre I, Chalmers AJ, Hellevik T. Martinez-Zubiaurre I, et al. Front Immunol. 2018 Jul 26;9:1679. doi: 10.3389/fimmu.2018.01679. eCollection 2018. Front Immunol. 2018. PMID: 30105016 Free PMC article. Review. - Tumor Vessels Fuel the Fire in Glioblastoma.
Rosińska S, Gavard J. Rosińska S, et al. Int J Mol Sci. 2021 Jun 17;22(12):6514. doi: 10.3390/ijms22126514. Int J Mol Sci. 2021. PMID: 34204510 Free PMC article. Review.
References
- Singh M, Ferrara N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nat Biotechnol 2012; 30: 648–57. - PubMed
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. . Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials