Adiponectin inhibits tumor necrosis factor-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation - PubMed (original) (raw)
Adiponectin inhibits tumor necrosis factor-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation
Yajing Wang et al. Circ Res. 2014.
Abstract
Rationale: Anti-inflammatory and vascular protective actions of adiponectin are well recognized. However, many fundamental questions remain unanswered.
Objective: The current study attempted to identify the adiponectin receptor subtype responsible for adiponectin's vascular protective action and investigate the role of ceramidase activation in adiponectin anti-inflammatory signaling.
Methods and results: Adiponectin significantly reduced tumor necrosis factor (TNF)α-induced intercellular adhesion molecule-1 expression and attenuated TNFα-induced oxidative/nitrative stress in human umbilical vein endothelial cells. These anti-inflammatory actions were virtually abolished by adiponectin receptor 1 (AdipoR1-), but not AdipoR2-, knockdown (KD). Treatment with adiponectin significantly increased neutral ceramidase (nCDase) activity (3.7-fold; P<0.01). AdipoR1-KD markedly reduced globular adiponectin-induced nCDase activation, whereas AdipoR2-KD only slightly reduced. More importantly, small interfering RNA-mediated nCDase-KD markedly blocked the effect of adiponectin on TNFα-induced intercellular adhesion molecule-1 expression. AMP-activated protein kinase-KD failed to block adiponectin-induced nCDase activation and modestly inhibited adiponectin anti-inflammatory effect. In contrast, in caveolin-1 KD (Cav1-KD) cells, >87% of adiponectin-induced nCDase activation was lost. Whereas adiponectin treatment failed to inhibit TNFα-induced intercellular adhesion molecule-1 expression, treatment with sphingosine-1-phosphate or SEW (sphingosine-1-phosphate receptor agonist) remained effective in Cav1-KD cells. AdipoR1 and Cav1 colocalized and coprecipitated in human umbilical vein endothelial cells. Adiponectin treatment did not affect this interaction. There is weak basal Cav1/nCDase interaction, which significantly increased after adiponectin treatment. Knockout of AdipoR1 or Cav1 abolished the inhibitory effect of adiponectin on leukocyte rolling and adhesion in vivo.
Conclusions: These results demonstrate for the first time that adiponectin inhibits TNFα-induced inflammatory response via Cav1-mediated ceramidase recruitment and activation in an AdipoR1-dependent fashion.
Keywords: adipokines; endothelial cells; inflammation; sphingolipids; vascular system injuries.
Figures
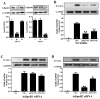
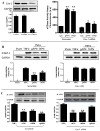
Figure 1. Effect of AdipoR1/AdipoR2 knoc`kdown upon APN’s anti-ICAM-1 effect
HUVEC were transfected with scramble or siRNA against AdipoR1/AdipoR2 to knockdown AdipoR expression (A). 48 hours after transfection, cells were pretreated with vehicle, gAPN or fAPN followed by TNFα treatment. Effect of APN upon TNFα-induced ICAM-1 expression (12 hours post-TNFα treatment) was determined in scramble (B), AdipoR1 siRNA (C) and AdipoR2 siRNA (D) transfected cells. *P<0.05, **P<0.01 vs. TNFα-treated animals without APN treatment.
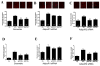
Figure 2. Effect of AdipoR1/AdipoR2 knockdown upon APN’s anti-oxidative and anti-nitrative effect
Cells were treated as described in Figure 1. Effect of APN upon TNFα-induced superoxide production and nitrotyrosine formation was determined in scramble (A/D), AdipoR1 (B/E) and AdipoR2 (C/F) transfected cells. *<0.05, **P<0.01 vs. TNFα-treated animals without APN treatment.
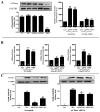
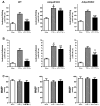
Figure 3. AdipoR1-dependent nCDase activation mediates APN anti-inflammatory action
(A): Transfection of HUVEC reduced nCDase expression (left panel) and blocked nCDase activation by APN (right panel); (B): Effect of AdipoR1 and AdipoR2 knockdown upon APN-induced nCDase activation; (C): Effect of nCDase knockdown upon the inhibitory action of APN upon TNFα-induced ICAM-1 expression. **P<0.01 vs. Con (A and B). *P<0.05, **P<0.01 vs. TNFα-treated animals without APN treatment (C).
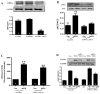
Figure 4. Role of AMPK in APN-induced nCDase activation
Transfection of HUVEC with AMPKα siRNA reduced AMPKα expression (A) and blocked gAPN-induced ACC phosphorylation (B). In contrast, APN-induced nCDase activation was not affected (C) and APN’s anti-TNFα (ICAM-1 expression, D) was only partially inhibited when AMPK expression was genetically inhibited. *P<0.05, **P<0.01 vs. respective control (Con).
Figure 5. Role of Cav1 in APN-induced nCDase activation and anti-inflammatory action
HUVEC Cav1 expression was genetically inhibited by Cav1 siRNA (A, left panel). Cav1 knockdown blocked APN-induced nCDase activation (A, right panel) and anti-inflammatory effects of APN (ICAM-1 expression, B). However, Cav1-KD had no significant effect upon anti-inflammatory effects of S1P and SEW2187 (C). **P<0.01 vs. respective control (A) or vs. TNFα-treated animals without APN treatment (B/C).
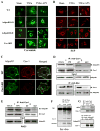
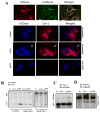
Figure 6. Effect of AdipoR1/AdipoR2 or Cav1 knockdown upon cellular levels of ceramide
(A) and S1P (B) after TNFα treatment in the presence and absence of gAPN. Representative photos from at least 5 repeated experiments. C: Cav1/AdipoR1 co-localization determined by immunofluorescent staining. Representative photos from at least 5 repeated experiments; D: Cav1/AdipoR1 interaction determined by co-immunoprecipitation in HUVEC. Cav1 was immunoprecipitated with antibody against Cav1 and samples were immunoblotted with antibody against AdipoR1 (upper panels). To immunoprecipitate AdipoR1, HUVEC were transfected with Myc-tagged AdipoR1 expressing vector and immunoprecipitated with antibody against Myc. Samples were then immunoblotted with antibody against Cav1 (lower panel). E: Cav1/AdipoR1 interaction determined by co-immunoprecipitation in RAEC. MUT=Re-expression in Cav1-KD cells of a mutated Cav1, in which 5 aromatic residues within the scaffolding domain responsible for Cav1 interaction with partner proteins were converted to alanine. F: Cav1/nCDase interaction determined by co-immunoprecipitation in aortic segment from mice treated with vehicle or gAPN. Representative blots from at least 5 repeated experiments; G: APN/Cav1 complex forming (upper panel) and lack of AdipoR2/Cav1 interaction determined by immunoprecipitation (lower panel). Representative blots from at least 5 repeated experiments.
Figure 7. Cellular distribution of nCDase without APN treatment
(A: top panel) and effect of APN treatment upon Cav1 and nCDase interaction in WT, Cav1-KD or AdipoR1KD HUVEC (A: confocal microscopy; B: immunoprecipitation). Note that APN-enhanced Cav1/nCDase interaction is blocked by AdipoR1, but not AdipoR2 knockdown. Cav1/AdipoR1/nCDase complex formation determined by immunoprecipitation (C/D). Representative images from at least 5 repeated experiments/experimental conditions.
Figure 8. Effect of AdipoR1/AdipoR2 knockout upon APN inhibition of TNFα-induced leukocyte rolling and adhesion
WT, AdipoR1KO and AdipoR2KO mice were pre-treated with vehicle or gAPN and leukocyte rolling and adhesion was observed using intravital microscopy following TNFα injection. The inhibitory effect of APN upon TNFα-induced leukocyte rolling (A) and adhesion (B) observed in WT mice was virtually abolished in AdipoR1 but not in AdipoR2 knockout mice. **P<0.01 vs. TNFα-treated animals without APN treatment; #P<0.05, ##P<0.01 vs. WT with the same treatment.
Similar articles
- High glucose/High Lipids impair vascular adiponectin function via inhibition of caveolin-1/AdipoR1 signalsome formation.
Liu GZ, Liang B, Lau WB, Wang Y, Zhao J, Li R, Wang X, Yuan Y, Lopez BL, Christopher TA, Xiao C, Ma XL, Wang Y. Liu GZ, et al. Free Radic Biol Med. 2015 Dec;89:473-85. doi: 10.1016/j.freeradbiomed.2015.09.005. Epub 2015 Oct 8. Free Radic Biol Med. 2015. PMID: 26453924 Free PMC article. - Loss of caveolin-1 and adiponectin induces severe inflammatory lung injury following LPS challenge through excessive oxidative/nitrative stress.
Cai L, Yi F, Dai Z, Huang X, Zhao YD, Mirza MK, Xu J, Vogel SM, Zhao YY. Cai L, et al. Am J Physiol Lung Cell Mol Physiol. 2014 Mar 15;306(6):L566-73. doi: 10.1152/ajplung.00182.2013. Epub 2014 Jan 17. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24441873 Free PMC article. - Adiponectin-AdipoR1/2-APPL1 signaling axis suppresses human foam cell formation: differential ability of AdipoR1 and AdipoR2 to regulate inflammatory cytokine responses.
Tian L, Luo N, Zhu X, Chung BH, Garvey WT, Fu Y. Tian L, et al. Atherosclerosis. 2012 Mar;221(1):66-75. doi: 10.1016/j.atherosclerosis.2011.12.014. Epub 2011 Dec 22. Atherosclerosis. 2012. PMID: 22227293 Free PMC article. - Adiponectin-Resistance in Obesity.
Engin A. Engin A. Adv Exp Med Biol. 2017;960:415-441. doi: 10.1007/978-3-319-48382-5_18. Adv Exp Med Biol. 2017. PMID: 28585210 Review. - Adiponectin Regulation and Function.
Fang H, Judd RL. Fang H, et al. Compr Physiol. 2018 Jun 18;8(3):1031-1063. doi: 10.1002/cphy.c170046. Compr Physiol. 2018. PMID: 29978896 Review.
Cited by
- Metabolic Messengers: Adiponectin.
Straub LG, Scherer PE. Straub LG, et al. Nat Metab. 2019 Mar;1(3):334-339. doi: 10.1038/s42255-019-0041-z. Epub 2019 Mar 14. Nat Metab. 2019. PMID: 32661510 Free PMC article. Review. - Adipokines in multiple sclerosis patients are related to clinical and radiological measures.
Loonstra FC, Falize KF, de Ruiter LRJ, Schoonheim MM, Strijbis EMM, Killestein J, de Vries HE, Uitdehaag BMJ, Rijnsburger M. Loonstra FC, et al. J Neurol. 2023 Apr;270(4):2018-2030. doi: 10.1007/s00415-022-11519-8. Epub 2022 Dec 23. J Neurol. 2023. PMID: 36562851 Free PMC article. - Adipokines as Immune Cell Modulators in Multiple Sclerosis.
Rijnsburger M, Djuric N, Mulder IA, de Vries HE. Rijnsburger M, et al. Int J Mol Sci. 2021 Oct 7;22(19):10845. doi: 10.3390/ijms221910845. Int J Mol Sci. 2021. PMID: 34639186 Free PMC article. Review. - ZFP36L1 and AUF1 Induction Contribute to the Suppression of Inflammatory Mediators Expression by Globular Adiponectin via Autophagy Induction in Macrophages.
Shrestha A, Pun NT, Park PH. Shrestha A, et al. Biomol Ther (Seoul). 2018 Sep 1;26(5):446-457. doi: 10.4062/biomolther.2018.078. Biomol Ther (Seoul). 2018. PMID: 30001609 Free PMC article. - Cardiovascular Adiponectin Resistance: The Critical Role of Adiponectin Receptor Modification.
Wang Y, Ma XL, Lau WB. Wang Y, et al. Trends Endocrinol Metab. 2017 Jul;28(7):519-530. doi: 10.1016/j.tem.2017.03.004. Epub 2017 May 1. Trends Endocrinol Metab. 2017. PMID: 28473178 Free PMC article. Review.
References
- Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. - PubMed
- Calles-Escandon J, Cipolla M. Diabetes and Endothelial Dysfunction: A Clinical Perspective. Endocr Rev. 2001;22:36–52. - PubMed
- Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, Ueda S, Shimomura I, Funahashi T, Matsuzawa Y. Hypoadiponectinemia Is Closely Linked to Endothelial Dysfunction in Man. J Clin Endocrinol Metab. 2003;88:3236–3240. - PubMed
- Tan KCB, Xu A, Chow WS, Lam MCW, Ai VHG, Tam SCF, Lam KSL. Hypoadiponectinemia Is Associated with Impaired Endothelium-Dependent Vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. - PubMed
- Maruyoshi H, Kojima S, Otsuka F, Funahashi T, Kaikita K, Sugiyama S, Sakamoto T, Yoshimura M, Shimomura I, Ogawa H. Hypoadiponectinemia is associated with coronary artery spasm in men. Circ J. 2005;69:1154–1156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 OD010408/OD/NIH HHS/United States
- HL-096686/HL/NHLBI NIH HHS/United States
- R01 HL096686/HL/NHLBI NIH HHS/United States
- HL-63828/HL/NHLBI NIH HHS/United States
- R01 HL063828/HL/NHLBI NIH HHS/United States
- T32 HL091804/HL/NHLBI NIH HHS/United States
- R01 DK096521/DK/NIDDK NIH HHS/United States
- 5R01DK064344/DK/NIDDK NIH HHS/United States
- P01 HL108806/HL/NHLBI NIH HHS/United States
- R01 DK064344/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous