Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance - PubMed (original) (raw)
. 2014 Mar 6;14(3):329-41.
doi: 10.1016/j.stem.2013.12.016. Epub 2014 Jan 16.
Markus Reschke 1, Riccardo Taulli 1, Olga Pozdnyakova 2, Kerri Burgess 1, Parul Bhargava 1, Kimberly Straley 3, Rahul Karnik 4, Alexander Meissner 4, Donald Small 5, Shinsan M Su 3, Katharine Yen 3, Jiangwen Zhang 6, Pier Paolo Pandolfi 7
Affiliations
- PMID: 24440599
- PMCID: PMC4380188
- DOI: 10.1016/j.stem.2013.12.016
Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance
Lev M Kats et al. Cell Stem Cell. 2014.
Abstract
Mutations in the metabolic enzymes isocitrate dehydrogenase-1 (IDH1) and IDH2 that produce the oncometabolite D-2-hydroxyglutarate (2-HG) occur frequently in human acute myeloid leukemia (AML). 2-HG modulates numerous biological pathways implicated in malignant transformation, but the contribution of mutant IDH proteins to maintenance and progression of AML in vivo is currently unknown. To answer this crucial question we have generated transgenic mice that express IDH2(R140Q) in an on/off- and tissue-specific manner using a tetracycline-inducible system. We found that IDH2(R140Q) can cooperate with overexpression of HoxA9 and Meis1a and with mutations in FMS-like tyrosine kinase 3 (FLT3) to drive acute leukemia in vivo. Critically, we show that genetic deinduction of mutant IDH2 in leukemic cells in vivo has profound effects on their growth and/or maintenance. Our data demonstrate the proto-oncogenic role of mutant IDH2 and support its relevance as a therapeutic target for the treatment of human AML.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
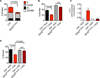
Figure 1. IDH2R140Q–t Mice Demonstrate Extramedullary Hematopoiesis Characterized by Spleen Enlargement and Expansion of Hematopoietic Stem/Progenitor Cells
(A) Schematic depicting the strategy for generation of an inducible IDH2R140Q allele in the mouse. Following Flp-mediated recombination, the IDH2R140Q cDNA flanked by a tetracycline response element (TRE) and a protamine-1 poly-A cassette is integrated into the ColA1 locus. The PGK promoter drives hygromycin resistance, allowing selection of integrants. (B) 2-HG in BM MNCs measured by LC-MS following 8 weeks of transgene induction. (C) Representative flow cytometry analysis of bone marrow mononuclear cells and splenocytes from mice treated with doxycycline for 8 weeks or 7 months. Numbers indicate cells within the gate as a percentage of all living cells in the sample. (D) Spleen weights of mice following 8 weeks of doxycycline treatment. (E) Percentage of cKit+Sca1+Lin− (KSL) cells in the spleens of mice following 8 weeks of doxycycline treatment. (F) Percentage of KSL cells in the bone marrow of mice following 7 months of doxycycline treatment. (G) Percentage of CD48−CD150+KSL hematopoietic stem cells in the bone marrow of mice following 7 months of doxycycline treatment. Error bars, mean ± SEM; p value calculated using a two-tailed unpaired Student’s t test. See also Figure S1.
Figure 2. IDH2R140Q–t Induces a Block of Erythroid Differentiation in KSL Cells That Is Reversible by Genetic Deinduction of the Transgene and by a Specific Inhibitor of Mutant IDH2
(A) Methylcellulose CFU-GEMM assay using KSL cells sorted from IDH2R140Q–t or control mice following 8 weeks of doxycycline treatment. Doxycycline was added to the media to maintain transgene expression throughout the differentiation process (n = 7 biological replicates/group). (B) Methylcellulose CFU-GEMM assay done as in (A) but with or without adding doxycycline to the medium (n = 3 biological replicates/group). (C and D) Methylcellulose CFU-GEMM assay done as in (A), with doxycycline in the medium and with or without the mutant IDH2 inhibitor AGI-6780. 2-HG was quantified by LC-MS in cells collected from the assay after 7 days of culture. b.q.l., below quantitative limit (C). Colonies were scored (n = 4 biological replicates/ group) in (D). Error bars, mean ± SEM; p value calculated using a two-tailed unpaired Student’s t test. See also Figure S2.
Figure 3. Identification of a Transcriptional Signature Associated with the _IDH2R140Q_-Induced Block of Differentiation
(A and B) Microarray analysis of cells cultured for 3 days in the methylcellulose CFU-GEMM assay (n ≥ 3 biological replicates/group). Hierarchical clustering of significantly altered genes (p < 0.05, fold change >1.5) is shown. Green, downregulated in IDH2R140Q–t cells; red, upregulated in IDH2R140Q–t cells (A). Pathway enrichment analysis shows genes that are downregulated in IDH2R140Q–t cells (green) or upregulated (red) in IDH2R140Q–t cells. The significance of the enrichment for each pathway (based on the false discovery rate q value) is proportional to the size of the gray box (i.e., the larger the gray box, the more significant the enrichment) (B). (C) qRT-PCR analysis of cells cultured as in (A) and (B) but with or without AGI-6780. For each of the transcripts examined, pharmacological inhibition of 2-HG production at least partially reverses transcriptional changes in IDH2R140Q–t cells. Error bars, mean ± SEM. See also Figure S3 and Tables S1, S2, and S3.
Figure 4. Continued Expression of IDH2R140Q Is Required for Proliferation and/or Maintenance of _HoxA9/Meis1a_–Transformed Leukemic Cells
(A) Schematic outlining the strategy for randomized deinduction experiments. (B) Representative FACS plots of bone marrow from secondary recipients untreated or treated with doxycycline. Continued expression of IDH2R140Q is essential for proliferation and/or maintenance of GFP/YFP+ leukemic cells and the onset of leukemia. (C) Percentage of GFP/YFP+ cells in the bone marrow of secondary recipients untreated or treated with doxycycline (n = 5 recipients on doxycycline, n = 3 recipients off doxycycline). (D) PCR on genomic DNA isolated from BM MNCs of doxycycline-treated and -untreated recipients using primers specific for MSCV-HoxA9-GFP and MSCV-Meis1a–YFP. Vector (i.e., MSCV-HoxA9-GFP or MSCV-Meis1a–YFP, respectively) DNA (V) and wild-type C57BL/6 genomic DNA (G) were used as controls. (E) Percentage of GFP/YFP+ cells in the bone marrow of secondary recipients untreated or treated with doxycycline at various time points following transplantation. Randomized deinduction was performed 2 weeks after transplantation (n = 2–3 recipients/time point/treatment). (F) Percentage of blasts in the bone marrow of secondary recipients untreated or treated with doxycycline at various time-points following transplantation. Randomized deinduction was performed 2 weeks after transplantation (n = 2–3 recipients/time point/treatment). Blasts were scored on May-Grunwald-Giemsa-stained bone marrow cytospins. See also Figure S4.
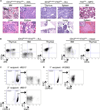
Figure 5. IDH2R140Q and Flt3ITD Cooperate in Leukemia Initiation
(A) Representative H&E staining of tissue sections from IDH2 R140Q–t;Flt3ITD compound transgenic animals and an Flt3ITD control animal. Compound transgenic animals develop acute myeloid (AML-like) and lymphoid (ALL-like) malignancies whereas_Flt3ITD_ animals develop myeloproliferative disease (MPD). Left panels: a compound transgenic animal diagnosed with AML-like disease. Apoptotic debris and numerous tingible body macrophages (magnified in inset, upper left panel) characteristic of highly proliferative disease are evident throughout. Infiltration of intermediate-sized blast-like immature mononuclear cells (magnified in inset, upper right panel) is evident in the red pulp of the spleen, the liver, and kidney. Middle panels: a compound transgenic animal diagnosed with ALL-like disease. T lymphoblasts (confirmed by immunohistochemical staining using anti-CD3ε antibody, not shown) with high-grade morphology are present in the thymus, spleen, liver, and lung. Numerous mitoses are present, characteristic of a rapidly proliferative disease. Right panels: MPD in an Flt3ITD animal with a hypercellular bone marrow with myeloid maturation and increased myeloid cells in the spleen. Original magnification, 600×. (B) Examples of FACS plots demonstrating the types of acute leukemia diagnosed in IDH2R140Q–t;Flt3ITD compound transgenic animals. Left panels: presence of CD3ε+cKit+ lymphoblasts in the thymus and bone marrow; cells in the thymus are exclusively CD8+CD4−. Right panels: cells in the bone marrow are almost exclusively Mac1+/low and a population of Mac1lowcKit+ immature cells is evident; in the thymus of the same animal, CD3ε−CD8+ lymphoblasts are present. (C and D) Primary AML (BM MNCs from donor #5517) and ALL (thymocytes from donor #12063) cells recapitulate a lethal leukemia in transplanted recipients treated with doxycycline. FACS plots demonstrate the overwhelming majority of donor-derived (CD45.2+) cells in the bone marrow of moribund recipients and the loss of lineage markers (Mac1 and CD3ε, respectively) upon serial transplantation. See also Figure S5.
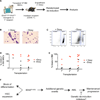
Figure 6. Mutant IDH2 Contributes to Leukemia Initiation and Maintenance in the Context of a Cooperating Flt3ITD Lesion
(A) Schematic outlining the strategy for randomized deinduction experiments using AML cells from recipient #5517. (B) May-Grunwald-Giemsa-stained bone marrow cytospins showing presence of leukemic blasts and differentiated myeloid cells in doxycycline-treated and -untreated recipients, respectively. (C) Examples of FACS plots showing reduced donor chimerism in untreated versus doxycycline-treated recipients based on differential staining for the cell surface markers CD45.1/CD45.2. (D and E) Genetic deinduction of IDH2R140Q expression by withdrawal of doxycycline has a profound effect on the proliferation and/or differentiation of leukemic blasts in vivo. Percentage of donor-derived (CD45.2+) cells (D) and blasts (E) in the bone marrow of primary, secondary, or tertiary recipients untreated or treated with doxycycline is shown. Blasts were scored on May-Grunwald-Giemsa-stained bone marrow cytospins. Randomized deinduction was performed 3 days after transplantation. (F) Model for the role of _IDH2R140_Q in primary HSPCs and in the maintenance of leukemia. See also Figure S5.
Similar articles
- IDH2 and NPM1 Mutations Cooperate to Activate Hoxa9/Meis1 and Hypoxia Pathways in Acute Myeloid Leukemia.
Ogawara Y, Katsumoto T, Aikawa Y, Shima Y, Kagiyama Y, Soga T, Matsunaga H, Seki T, Araki K, Kitabayashi I. Ogawara Y, et al. Cancer Res. 2015 May 15;75(10):2005-16. doi: 10.1158/0008-5472.CAN-14-2200. Epub 2015 Mar 20. Cancer Res. 2015. PMID: 25795706 - Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition.
Chen C, Liu Y, Lu C, Cross JR, Morris JP 4th, Shroff AS, Ward PS, Bradner JE, Thompson C, Lowe SW. Chen C, et al. Genes Dev. 2013 Sep 15;27(18):1974-85. doi: 10.1101/gad.226613.113. Genes Dev. 2013. PMID: 24065765 Free PMC article. - Mutant Idh2 Cooperates with a NUP98-HOXD13 Fusion to Induce Early Immature Thymocyte Precursor ALL.
Goldberg L, Negi V, Chung YJ, Onozawa M, Zhu YJ, Walker RL, Pierce R, Patel DP, Krausz KW, Gonzalez FJ, Goodell MA, Rodriguez BAT, Meltzer PS, Aplan PD. Goldberg L, et al. Cancer Res. 2021 Oct 1;81(19):5033-5046. doi: 10.1158/0008-5472.CAN-21-1027. Epub 2021 Jul 28. Cancer Res. 2021. PMID: 34321240 Free PMC article. - The role of mutant IDH1 and IDH2 inhibitors in the treatment of acute myeloid leukemia.
Nassereddine S, Lap CJ, Haroun F, Tabbara I. Nassereddine S, et al. Ann Hematol. 2017 Dec;96(12):1983-1991. doi: 10.1007/s00277-017-3161-0. Epub 2017 Oct 31. Ann Hematol. 2017. PMID: 29090344 Review. - Acute myeloid leukemia with IDH1 and IDH2 mutations: 2021 treatment algorithm.
Issa GC, DiNardo CD. Issa GC, et al. Blood Cancer J. 2021 Jun 3;11(6):107. doi: 10.1038/s41408-021-00497-1. Blood Cancer J. 2021. PMID: 34083508 Free PMC article. Review.
Cited by
- Cancer Stem Cells: Metabolic Characterization for Targeted Cancer Therapy.
Kaur J, Bhattacharyya S. Kaur J, et al. Front Oncol. 2021 Nov 5;11:756888. doi: 10.3389/fonc.2021.756888. eCollection 2021. Front Oncol. 2021. PMID: 34804950 Free PMC article. Review. - Characterization of cancer-associated IDH2 mutations that differ in tumorigenicity, chemosensitivity and 2-hydroxyglutarate production.
Kotredes KP, Razmpour R, Lutton E, Alfonso-Prieto M, Ramirez SH, Gamero AM. Kotredes KP, et al. Oncotarget. 2019 Apr 12;10(28):2675-2692. doi: 10.18632/oncotarget.26848. eCollection 2019 Apr 12. Oncotarget. 2019. PMID: 31105869 Free PMC article. - MIR142 Loss-of-Function Mutations Derepress ASH1L to Increase HOXA Gene Expression and Promote Leukemogenesis.
Trissal MC, Wong TN, Yao JC, Ramaswamy R, Kuo I, Baty J, Sun Y, Jih G, Parikh N, Berrien-Elliott MM, Fehniger TA, Ley TJ, Maillard I, Reddy PR, Link DC. Trissal MC, et al. Cancer Res. 2018 Jul 1;78(13):3510-3521. doi: 10.1158/0008-5472.CAN-17-3592. Epub 2018 May 3. Cancer Res. 2018. PMID: 29724719 Free PMC article. - Genetic dissection of leukemia-associated IDH1 and IDH2 mutants and D-2-hydroxyglutarate in Drosophila.
Reitman ZJ, Sinenko SA, Spana EP, Yan H. Reitman ZJ, et al. Blood. 2015 Jan 8;125(2):336-45. doi: 10.1182/blood-2014-05-577940. Epub 2014 Nov 14. Blood. 2015. PMID: 25398939 Free PMC article. - A pharmacogenomic approach validates AG-221 as an effective and on-target therapy in IDH2 mutant AML.
Kats LM, Vervoort SJ, Cole R, Rogers AJ, Gregory GP, Vidacs E, Li J, Nagaraja R, Yen KE, Johnstone RW. Kats LM, et al. Leukemia. 2017 Jun;31(6):1466-1470. doi: 10.1038/leu.2017.84. Epub 2017 Mar 10. Leukemia. 2017. PMID: 28280273 No abstract available.
References
- Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, Pollock R, O’Donnell P, Grigoriadis A, Diss T, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224:334–343. - PubMed
- Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–28. - PubMed
- Chaturvedi A, Araujo Cruz MM, Jyotsana N, Sharma A, Yun H, Görlich K, Wichmann M, Schwarzer A, Preller M, Thol F, et al. Mutant IDH1 promotes leukemogenesis in vivo and can be specifically targeted in human AML. Blood. 2013;122:2877–2887. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous