Long-lived intestinal tuft cells serve as colon cancer-initiating cells - PubMed (original) (raw)
. 2014 Mar;124(3):1283-95.
doi: 10.1172/JCI73434.
Samuel Asfaha, Yoku Hayakawa, Yoshihiro Takemoto, Dana J Lukin, Andreas H Nuber, Anna Brandtner, Wanda Setlik, Helen Remotti, Ashlesha Muley, Xiaowei Chen, Randal May, Courtney W Houchen, James G Fox, Michael D Gershon, Michael Quante, Timothy C Wang
- PMID: 24487592
- PMCID: PMC3934168
- DOI: 10.1172/JCI73434
Long-lived intestinal tuft cells serve as colon cancer-initiating cells
C Benedikt Westphalen et al. J Clin Invest. 2014 Mar.
Abstract
Doublecortin-like kinase 1 protein (DCLK1) is a gastrointestinal tuft cell marker that has been proposed to identify quiescent and tumor growth-sustaining stem cells. DCLK1⁺ tuft cells are increased in inflammation-induced carcinogenesis; however, the role of these cells within the gastrointestinal epithelium and their potential as cancer-initiating cells are poorly understood. Here, using a BAC-CreERT-dependent genetic lineage-tracing strategy, we determined that a subpopulation of DCLK1⁺ cells is extremely long lived and possesses rare stem cell abilities. Moreover, genetic ablation of Dclk1 revealed that DCLK1⁺ tuft cells contribute to recovery following intestinal and colonic injury. Surprisingly, conditional knockdown of the Wnt regulator APC in DCLK1⁺ cells was not sufficient to drive colonic carcinogenesis under normal conditions; however, dextran sodium sulfate-induced (DSS-induced) colitis promoted the development of poorly differentiated colonic adenocarcinoma in mice lacking APC in DCLK1⁺ cells. Importantly, colonic tumor formation occurred even when colitis onset was delayed for up to 3 months after induced APC loss in DCLK1⁺ cells. Thus, our data define an intestinal DCLK1⁺ tuft cell population that is long lived, quiescent, and important for intestinal homeostasis and regeneration. Long-lived DCLK1⁺ cells maintain quiescence even following oncogenic mutation, but are activated by tissue injury and can serve to initiate colon cancer.
Figures
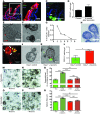
Figure 1. DCLK1 labels long-lived epithelial tuft cells.
(A) Representative LacZ staining of tuft cells in the gastrointestinal tract of Dclk1 R26LacZ mice 24 hours after tamoxifen administration. (B) Representative immunofluorescence staining for DCLK1 (red) in Dclk1-CreGFP mice. (C) Representative LacZ staining of tuft cells in the gastrointestinal tract of Dclk1 R26LacZ mice up to 18 months after tamoxifen administration. (D) Quantification of labeled tuft cells in Dclk1 R26LacZ mice at different time points after tamoxifen treatment (n >5). (E) Quantification of DCLK1+ cells in Dclk1 R26-TGFP × Dclk1flox/WT mice compared with controls 8 weeks after tamoxifen administration (n = 3). ***P < 0.001. (F and G) Representative immunofluorescence staining for DCLK1 (red) and BrdU (green) in the small intestine and colon. (H) Quantification of BrdU-labeled DCLK1+ tuft cells after 1, 3, and 6 months of continuous BrdU administration (n = 3). Scale bars: 10 μm (B), 20 μm (A and C), and 50 μm (C, top two right panels; F and G).
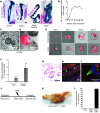
Figure 2. DCLK1+ tuft cells are derived from LGR5 stem cells and require neural input for survival.
(A and B) Representative immunofluorescence for DCLK1 (green) in Lgr5-CreERT-IRES-GFP R26tdTom mice. Scale bars: 50 μm (A) and 20 μm (B). (C) Representative immunofluorescence for DCLK1 (red) in Lgr5-CreERT-IRES-GFP × Atohflox/flox mice. Scale bar: 10 μm. (D) Quantification of DCLK1+ tuft cells in GFP-positive crypts in Lgr5-CreERT-IRES-GFP Atohflox/flox mice 2 months after administration of tamoxifen (n = 3). (E and F) Representative images of intestinal organoids derived from Dclk1 R26tdTom mice 24 hours (E) and 7 days (F) after isolation. Scale bars: 20 μm (E) and 25 μm (F). (G) Quantification of recombined cells in intestinal organoids derived from Dclk1 R26tdTom mice (n = 3). (H) IHC for DCLK1 in paraffin-embedded intestinal organoids 14 days after isolation. Scale bar: 20 μm. (I) Reconstruction of cocultured colonic organoids (red) and primary neurons (green) imaged with 2-photon microscopy. (J and K) Colonic organoids derived from Dclk1 R26tdTom mice cultured in the absence (J) and presence (K) of GFP-positive neurons after 7 days in culture. Scale bars: 50 μm (I–K). (L) Quantification of recombined cells in colonic organoids derived from Dclk1 R26tdTom in the absence and presence of neurons 14 days after isolation. (M–P) Colonic organoids derived from Dclk1 R26-DTA mice cultured in the presence (N and P) and absence (M and O) of neurons. 4-OH-tamoxifen was added to induce the expression of DTA in DCLK+ cells (O and P). Scale bars: 100 μm (M–P). (Q) Quantification of colonic organoids in the presence (+ Neurons) and absence of neurons and after DCLK1+ cell ablation (+ Tam). Results are shown as percentages and normalized to control (– Tam). (R) Size of colonic organoids in the presence (+ Neurons) and absence of neurons and after DCLK1+ cell ablation (+ Tam) (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 3. Intestinal DCLK1+ cells are involved in tissue homeostasis and play a critical role in regeneration.
(A and C) IHC for DCLK1 and Ki67 in the small intestine (A) and colon (C) of control mice without diphtheria toxin (– DTX) and after ablation of DCLK1+ cells in Dclk1 R26-iDTR mice (+ DTX). (B and D) Quantification of Ki67 cells in the small intestine (A) and colon (C) of Dclk1 R26-iDTR after treatment with diphtheria toxin compared with controls (n = 3). (E) Overall survival of Dclk1 R26-DTA (n = 5) and control mice (n = 5) after whole-body irradiation. (F–H) Representative H&E-stained sections of the regenerating epithelium from control (F) and Dclk1 R26-DTA mice (G and H) 5–7 days after irradiation. (I) Overall survival of Dclk1 R26-DTA (n = 5) and control mice (n = 5) after DSS treatment. (J–L) Representative H&E-stained sections from control (J) and Dclk1 R26-DTA mice (K and L) 7 days after DSS treatment. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars: 20 μm (C). Original magnification, x100 (A, F–H, and J–L).
Figure 4. Quiescent DCLK1+ cells serve as colon cancer–initiating cells.
(A) LacZ staining of Dclk1 R26LacZ mice showing traced crypts in the stomach, small intestine, and colon. Scale bars: 50 μm. (B) Quantification of traced crypts in Dclk1 R26LacZ mice. (C and D) Intestinal organoids derived from Dclk1 R26tdTom mice in the presence of Wnt3a at baseline (C) and after 2 weeks (D) in culture. Scale bars: 50 μm (C) and 25 μm (D). (E) DCLK1+ cells from the small intestine (upper panels) and colon (lower panels) of Dclk1 R26tdTom mice were sorted based on red fluorescent protein (RFP) expression (see also Supplemental Figure 1) and cultured in vitro for 7 days in the presence and absence of Wnt3a. Original magnification, x100. (F) Quantification of organoid formation in the presence and absence of Wnt3a (n = 3). (G) LacZ staining of the colon of a Dclk1 R26LacZ Apcflox/flox mouse 14 months after tamoxifen treatment. Scale bar: 25 μm. (H and I) IHC for β-gal (green) and β-catenin (red) in Dclk1 R26LacZ Apcflox/flox mice after tamoxifen treatment. (I) Representative image of a single recombined DCLK1+ cell without nuclear translocation of β-catenin. Scale bars: 20 μm (H) and 10 μm (I). (J) Experimental setup for DSS treatment in Dclk1 R26LacZ Apcflox/flox mice. (K) Gross pathology of resulting tumors in Dclk1 R26LacZ Apcflox/flox mice. Scale bar: 0.5 inches. (L) Quantification of tumor incidence in Dclk1 R26LacZ Apcflox/flox mice after treatment with tamoxifen, DSS only, and tamoxifen plus DSS (n ≥5 mice/group).
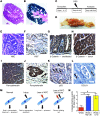
Figure 5. DCLK1+ cells give rise to poorly differentiated colorectal cancer.
(A) H&E staining of a colonic tumor in a Dclk1 Apcflox/flox mouse after DSS treatment. (B) LacZ staining of the tumor depicted in A. Original magnification, x40 (A and B). (C) Experimental setup for delayed DSS treatment studies. (D) Macroscopic appearance of resulting tumors after delayed administration of DSS. (E) H&E staining of resulting tumors in a Dclk1 Apcflox/flox mice. Scale bar: 50 μm. (F) IHC for Ki67 of the same tumor showing elevated numbers of Ki67+ cells. Scale bar: 100 μm. (G) IHC for β-catenin in an unaffected area of the colon. β-Catenin shows membrane association. Scale bar: 50 μm. (H) IHC for β-catenin in a resulting tumor showing strong cytoplasmic and nuclear staining. Scale bar: 50 μm. (I) IHC for pancytokeratin showing the complex architecture of the tumor. (J) IHC for pancytokeratin showing a malignant gland invading the surrounding stroma. Arrow shows budding tumor cells. Scale bar: 25 μm. (K) IHC for p53. Arrows show scattered cells with nuclear p53 staining. Scale bar: 50 μm. (L) IHC for DCLK1. Rare DCLK1+ cells (arrows) can be found in the tumor. Scale bar: 25 μm. (M) Proposed model for the role of DCLK1+ tuft cells in homeostasis and injury and as cancer-initiating cells. (N) In vitro lineage-tracing events in the presence of recombinant Wnt3a (red bar), macrophage-conditioned media supplemented with Wnt3a (blue bar), and medium supplemented with recombinant Wnt3a and IL-1β (yellow bar).
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS015547/NS/NINDS NIH HHS/United States
- CAPMC/ CIHR/Canada
- P30 ES002109/ES/NIEHS NIH HHS/United States
- R01 NS012969/NS/NINDS NIH HHS/United States
- R01 DK097016/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials