Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition? - PubMed (original) (raw)
Review
Stress, glucocorticoid receptors, and adult neurogenesis: a balance between excitation and inhibition?
Dirk-Jan Saaltink et al. Cell Mol Life Sci. 2014 Jul.
Abstract
Adult neurogenesis, the birth of new neurons in the mature brain, has attracted considerable attention in the last decade. One of the earliest identified and most profound factors that affect adult neurogenesis both positively and negatively is stress. Here, we review the complex interplay between stress and adult neurogenesis. In particular, we review the role of the glucocorticoid receptor, the main mediator of the stress response in the proliferation, differentiation, migration, and functional integration of newborn neurons in the hippocampus. We review a multitude of mechanisms regulating glucocorticoid receptor activity in relationship to adult neurogenesis. We postulate a novel concept in which the level of glucocorticoid receptor expression directly regulates the excitation-inhibition balance, which is key for proper neurogenesis. We furthermore argue that an excitation-inhibition dis-balance may underlie aberrant functional integration of newborn neurons that is associated with psychiatric and paroxysmal brain disorders.
Figures
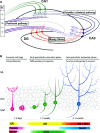
Fig. 1
Overview of the hippocampal formation and adult neurogenesis. a Schematic representation of the tri-synaptic pathway. Axons derived from the entorhinal cortex (EC) layer II projects via the perforant pathway (purple) towards the dendrites of the DG granule cells (red). The perforant pathway projects also towards CA3 were they end in boutons, which contact dendrites of pyramidal cells (blue). Mossy fibers (red) originating in the DG granule cells project to the same pyramidal cells in the CA3. Via the Schaffer collateral pathway (blue) the CA3 projects towards pyramidal neurons (green) in the CA1, which also receive input from the EC layer III. CA1 pyramidal neurons project (green) towards layer IV of the same EC. Only in the DG, new-born granule cells (yellow) integrate into this network. b Adult neurogenesis can be divided in three main stages: the precursor cell stage, the early postmitotic maturation phase and the late mitotic maturation phase. Stem cells proliferate in the sub-granular zone (SGZ) where after NPCs migrate into the granular cell layer (GCL). During the late postmitotic phase, the newborn neurons develop dendritic trees protruding into the molecular layer (ML). Different stages are characterized by expression of specific markers. Note that the GR is expressed in radial glia cells, but not early precursor cells. For further details, see main text
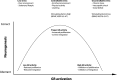
Fig. 2
Relationship between GR activity and neurogenesis GR activation exerts both positive and negative effects on neurogenesis. We propose an inverted U-shaped model in which there is a relationship between the amount of GR activation and neurogenesis. Low levels of stress seen in animals kept in a poor environment or with a sedentary lifestyle induce low levels of proliferation and maturation. Controllable stress, like enriched environments, physical activity, and learning, coincides with increased levels of GR activation and is associated with increased cell proliferation and correct integration of mature neurons. Too much GR activation as seen during uncontrollable stress negatively affects proliferation and neuronal integration. Neurogenesis-controlling molecular factors, like BDNF, VEGF, and 5-HT signaling, are regulated by low–high GR activity in opposite directions
Fig. 3
GR knockdown stimulates differentiation of post-mitotic neuronal precursor cells. GR knockdown increases the number of dendritic mature mushroom spines (1), axonal bouton size (2), and number of mini-excitatory postsynaptic currents (mEPSC; 4). GR knockdown also leads to more complex dendritic arbors (2) and ectopically located new-born granule cells (not illustrated). After [108]
Fig. 4
The excitation–inhibition balance determines the number and final positioning of newborn granule cells. Excessive excitation, as is the case after seizures, stroke, and GR knockdown, leads to an increase and ectopic location of newborn granule cells. Reversely, excessive inhibition leads to reduced numbers of newborn cells. Although suggested in this cartoon, it is unknown whether newborn granule cells are ectopically located in the GCL. For further details, see main text
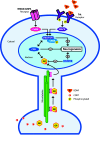
Fig. 5
Hypothetical model illustrating possible molecular mechanisms underlying the action of the glucocorticoid receptor in the excitation-inhibition balance in NPCs. Activation of NMDA and AMPA receptors lead to an intracellular signaling cascade leading to the activation of the pro-neurogenic transcription factors CREB and zif68/EGR via CaMK. Likewise, binding of neurotrophins (e.g., BDNF) to Trks leads to activation of CREB and zif68/egr1 via the MAPK pathway. CORT-activated GR is retrograde transported by doublecortin-like (DCL) along the microtubules towards the nucleus. GR may interact with p-CREB by interaction with co-factors such as CREB binding protein (CPB). The net result of this GR-CPB-CREB interaction is the decreased CREB activity and subsequent inhibition of neurogenesis. Pro-neurogenic miR-124 represses GR activity, which may facilitate neuronal differentiation via NMDAR and/or Trk activation
Similar articles
- Glucocorticoids Suppress the Protective Effect of Cyclooxygenase-2-Related Signaling on Hippocampal Neurogenesis Under Acute Immune Stress.
Ma Y, Matsuwaki T, Yamanouchi K, Nishihara M. Ma Y, et al. Mol Neurobiol. 2017 Apr;54(3):1953-1966. doi: 10.1007/s12035-016-9766-9. Epub 2016 Feb 24. Mol Neurobiol. 2017. PMID: 26910812 - Adaptor Protein APPL2 Affects Adult Antidepressant Behaviors and Hippocampal Neurogenesis via Regulating the Sensitivity of Glucocorticoid Receptor.
Gao C, Chen X, Xu A, Cheng K, Shen J. Gao C, et al. Mol Neurobiol. 2018 Jul;55(7):5537-5547. doi: 10.1007/s12035-017-0785-y. Epub 2017 Sep 30. Mol Neurobiol. 2018. PMID: 28965332 - Circadian and ultradian glucocorticoid rhythmicity: Implications for the effects of glucocorticoids on neural stem cells and adult hippocampal neurogenesis.
Fitzsimons CP, Herbert J, Schouten M, Meijer OC, Lucassen PJ, Lightman S. Fitzsimons CP, et al. Front Neuroendocrinol. 2016 Apr;41:44-58. doi: 10.1016/j.yfrne.2016.05.001. Epub 2016 May 24. Front Neuroendocrinol. 2016. PMID: 27234350 Review. - Hormonal Regulation of Mammalian Adult Neurogenesis: A Multifaceted Mechanism.
Jorgensen C, Wang Z. Jorgensen C, et al. Biomolecules. 2020 Aug 6;10(8):1151. doi: 10.3390/biom10081151. Biomolecules. 2020. PMID: 32781670 Free PMC article. Review. - Stress-Related Dysfunction of Adult Hippocampal Neurogenesis-An Attempt for Understanding Resilience?
Leschik J, Lutz B, Gentile A. Leschik J, et al. Int J Mol Sci. 2021 Jul 8;22(14):7339. doi: 10.3390/ijms22147339. Int J Mol Sci. 2021. PMID: 34298958 Free PMC article. Review.
Cited by
- Anxiety and hippocampal neuronal activity: Relationship and potential mechanisms.
Ghasemi M, Navidhamidi M, Rezaei F, Azizikia A, Mehranfard N. Ghasemi M, et al. Cogn Affect Behav Neurosci. 2022 Jun;22(3):431-449. doi: 10.3758/s13415-021-00973-y. Epub 2021 Dec 6. Cogn Affect Behav Neurosci. 2022. PMID: 34873665 Review. - Increased Testosterone Decreases Medial Cortical Volume and Neurogenesis in Territorial Side-Blotched Lizards (Uta stansburiana).
LaDage LD, Roth TC 2nd, Downs CJ, Sinervo B, Pravosudov VV. LaDage LD, et al. Front Neurosci. 2017 Mar 1;11:97. doi: 10.3389/fnins.2017.00097. eCollection 2017. Front Neurosci. 2017. PMID: 28298883 Free PMC article. - Food restriction reduces neurogenesis in the avian hippocampal formation.
Robertson BA, Rathbone L, Cirillo G, D'Eath RB, Bateson M, Boswell T, Wilson PW, Dunn IC, Smulders TV. Robertson BA, et al. PLoS One. 2017 Dec 6;12(12):e0189158. doi: 10.1371/journal.pone.0189158. eCollection 2017. PLoS One. 2017. PMID: 29211774 Free PMC article. - Adult-born neurons from the dorsal, intermediate, and ventral regions of the longitudinal axis of the hippocampus exhibit differential sensitivity to glucocorticoids.
Levone BR, Codagnone MG, Moloney GM, Nolan YM, Cryan JF, O' Leary OF. Levone BR, et al. Mol Psychiatry. 2021 Jul;26(7):3240-3252. doi: 10.1038/s41380-020-0848-8. Epub 2020 Jul 24. Mol Psychiatry. 2021. PMID: 32709996 - The effects of peripheral hormone responses to exercise on adult hippocampal neurogenesis.
Kraemer RR, Kraemer BR. Kraemer RR, et al. Front Endocrinol (Lausanne). 2023 Nov 24;14:1202349. doi: 10.3389/fendo.2023.1202349. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38084331 Free PMC article. Review.
References
- Cajal Y, Ramón S. Degeneration and regeneration of the nervous system. London: Oxford University Press; 1928.
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. - PubMed
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources