In vivo activity of miR-34a mimics delivered by stable nucleic acid lipid particles (SNALPs) against multiple myeloma - PubMed (original) (raw)
. 2014 Feb 27;9(2):e90005.
doi: 10.1371/journal.pone.0090005. eCollection 2014.
Virginia Campani 2, Gabriella Misso 3, Maria Eugenia Gallo Cantafio 4, Annamaria Gullà 4, Umberto Foresta 4, Pietro Hiram Guzzi [ 5](#full-view-affiliation-5 "Department of Medical and Surgical Sciences, Laboratory of Bioinformatics Unit, "Salvatore Venuta" University Campus, Catanzaro, Italy."), Maria Castellano 3, Anna Grimaldi 3, Vincenzo Gigantino [ 6](#full-view-affiliation-6 "Pathology Unit, National Institute of Tumours of Naples "Pascale", Naples, Italy."), Renato Franco [ 6](#full-view-affiliation-6 "Pathology Unit, National Institute of Tumours of Naples "Pascale", Naples, Italy."), Sara Lusa 2, Mario Cannataro [ 5](#full-view-affiliation-5 "Department of Medical and Surgical Sciences, Laboratory of Bioinformatics Unit, "Salvatore Venuta" University Campus, Catanzaro, Italy."), Pierosandro Tagliaferri [ 1](#full-view-affiliation-1 "Department of Experimental and Clinical Medicine, Magna Graecia University and Medical Oncology Unit, Catanzaro, Italy ; T. Campanella Cancer Center, "Salvatore Venuta" University Campus, Catanzaro, Italy."), Giuseppe De Rosa 2, Pierfrancesco Tassone [ 7](#full-view-affiliation-7 "Department of Experimental and Clinical Medicine, Magna Graecia University and Medical Oncology Unit, Catanzaro, Italy ; T. Campanella Cancer Center, "Salvatore Venuta" University Campus, Catanzaro, Italy ; Sbarro Institute for Cancer Research and Molecular Medicine, Center for Biotechnology, College of Science and Technology, Temple University, Philadelphia, Pennsylvania, United States of America."), Michele Caraglia 8
Affiliations
- PMID: 24587182
- PMCID: PMC3937395
- DOI: 10.1371/journal.pone.0090005
In vivo activity of miR-34a mimics delivered by stable nucleic acid lipid particles (SNALPs) against multiple myeloma
Maria Teresa Di Martino et al. PLoS One. 2014.
Abstract
Multiple myeloma (MM) is a disease with an adverse outcome and new therapeutic strategies are urgently awaited. A rising body of evidence supports the notion that microRNAs (miRNAs), master regulators of eukaryotic gene expression, may exert anti-MM activity. Here, we evaluated the activity of synthetic miR-34a in MM cells. We found that transfection of miR-34a mimics in MM cells induces a significant change of gene expression with relevant effects on multiple signal transduction pathways. We detected early inactivation of pro-survival and proliferative kinases Erk-2 and Akt followed at later time points by caspase-6 and -3 activation and apoptosis induction. To improve the in vivo delivery, we encapsulated miR-34a mimics in stable nucleic acid lipid particles (SNALPs). We found that SNALPs miR-34a were highly efficient in vitro in inhibiting growth of MM cells. Then, we investigated the activity of the SNALPs miR-34a against MM xenografts in SCID mice. We observed significant tumor growth inhibition (p<0.05) which translated in mice survival benefits (p=0.0047). Analysis of miR-34a and NOTCH1 expression in tumor retrieved from animal demonstrated efficient delivery and gene modulation induced by SNALPs miR-34a in the absence of systemic toxicity. We here therefore provide evidence that SNALPs miR-34a may represent a promising tool for miRNA-therapeutics in MM.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
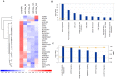
Figure 1. Whole Gene profiling perturbations induced by synthetic miR-34a.
A) Heatmap representation of the top 28 down- and up-regulated genes (P<0.001) following miR-34a or miR-NC transfection in SKMM-1 cells by Gene 1.0 ST array chip (Affymetrix) and DChip software. Data are presented row normalized (range from −3 to +3 standard deviations from median in expression). Genes that underwent a 1.5-fold change as compared to control, were selected and clustered. Assays performed in triplicate are shown. Ingenuity Pathway analysis of biological function annotation B) and canonical pathways C) for differential expressed gene (FC = +1.5) after miR-34a transfection respect to the miR-NC control. The bar graphs show pathways most modulated by miR-34a inhibitors as compared to control, based on statistical significance (P-value and ratio). The yellow line indicates the threshold of significance.
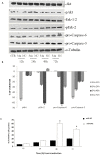
Figure 2. Effects of miR-34a replacement on survival pathways and apoptosis occurrence.
A) SKMM-1 cells were transfected with miR-34a (34a) or scramble miR-NC (NC) and after different times from the transfection were collected for Western blot analysis. Thereafter, the expression and phosphorylation of Erk, the activity and expression of Akt and pro-caspase-6 and -3 expression were evaluated after blotting with specific antibodies, as described in “Material and Methods”. The house-keeping protein α-tubulin was used as loading control. Each point is representative of 3 different evaluations performed in 3 different experiments. B) Scan of the bands associated with pErk-2 expression and Akt activity normalized for total Erk-2 or Akt expression, respectively, and of pro-caspase-3 and pro-caspase-6 expression, normalized with the housekeeping protein α-tubulin in SKMM-1 cells, was performed with ImageJ software. The intensities of the bands were expressed as % of changes based upon determination of arbitrary units (%, mean of three different experiments). Each point is the mean of 3 different evaluations performed in at least 3 different experiments. Bars, s.e.’s. C) SKMM-1 cells after transfection with miR-34a (34a) or scramble miR-NC (NC). The cells were collected after the indicated times from the transfection and apoptosis was evaluated with TUNEL assay by FACScan as described in “Materials and Methods”. Results are shown as percentage of apoptotic cells. Data are the average ‘SD of 3 independent experiments.
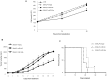
Figure 3. SNALPs formulated miR-34a has anti-proliferative activity against MM in vitro and in vivo.
A) Trypan blue exclusion assay of SKMM-1 cells treated with SNALP-encapsulated miR-34a or scramble oligonucleotides as control (NC). Analysis was performed by microscope Burker chamber counts and trypan blue exclusion assay. Averaged values of three independent experiments are plotted including ±SD. P-values calculated by Student’s t test, two-tailed, at 24 and 48 hours after transfection, are respectively: 0.001 and 0.02 versus SNALP empty or 0.0099 and 0.01 versus SNALP miR-NC. B) Mice carrying palpable subcutaneous SKMM-1 tumor xenografts were treated by intravenous tail vein injections with 20 µg for each treatment of miR-34a encapsulated into SNALPs. As control SNALPs incapsulating scramble miR-NC or empty were used. Caliper measurement of tumors were taken every 2 days from the day of the enrollment. Averaged tumor volumes of 4 mice per group are reported±SD. (*) indicate significant _P_-values (P<0.05). D) Survival curves (Kaplan-Meier) of treated mice show prolongation of survival after SNALP formulated miR-34a treatment compared to controls (log-rank test, P = 0.0047 and 0.002 SNALP miR-34a vs empty and miR-NC, respectively). Survival was evaluated from the first day of treatment until death or sacrifice.
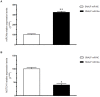
Figure 4. Effects induced by systemic delivery of miR-34a in MM xenografts.
miR-34a q-RT-PCR A) and q-RT-PCR of NOTCH1 mRNA expression B) at the end of observation of animal treatments with SNALP miR-34a formulation and SNALP miR-NC as control, in retrieved xenograft SKMM-1 tumors. The results are shown as average of miR-34a or NOTCH1 mRNA expression level after normalization with RNU44 or GAPDH, respectively, and ΔΔCt calculations. Data represent the average of 3 independent experiments ±SD. (*) P<0.05, (**) P<0.01.
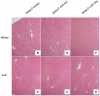
Figure 5. H&E staining of livers and kidney indicates absence of systemic toxicity.
Hematoxylin and eosin staining (40-fold magnification) of kidney and liver retrieved from SNALP empty (A, B), SNALP miR-NC (C, D) and SNALP miR-34a (E, F) treated mice, respectively. No significant damage was detected in the different groups of treatment. Representative image are shown.
Figure 6. SNALP miR-34a reduces Akt activation and induces apoptosis in MM in vivo.
TUNEL assay of SKMM-1 xenograft retrieved from SNALP miR-NC (A, B) and SNALP miR-34a (E, F) treated mice. The TUNEL positive cells are colored in brown. Representative image at 40-fold (A, E) and 60-fold (B, F) magnification are shown. p-Akt immunostaining SKMM-1 xenograft retrieved from SNALP miR-NC (C, D) and SNALP miR-34a (G, H) treated mice. Representative image at 40-fold (C, G) and 60-fold (D, H) magnification are shown.
Similar articles
- Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence.
Di Martino MT, Leone E, Amodio N, Foresta U, Lionetti M, Pitari MR, Cantafio ME, Gullà A, Conforti F, Morelli E, Tomaino V, Rossi M, Negrini M, Ferrarini M, Caraglia M, Shammas MA, Munshi NC, Anderson KC, Neri A, Tagliaferri P, Tassone P. Di Martino MT, et al. Clin Cancer Res. 2012 Nov 15;18(22):6260-70. doi: 10.1158/1078-0432.CCR-12-1708. Epub 2012 Oct 3. Clin Cancer Res. 2012. PMID: 23035210 Free PMC article. - Transferrin-conjugated SNALPs encapsulating 2'-O-methylated miR-34a for the treatment of multiple myeloma.
Scognamiglio I, Di Martino MT, Campani V, Virgilio A, Galeone A, Gullà A, Gallo Cantafio ME, Misso G, Tagliaferri P, Tassone P, Caraglia M, De Rosa G. Scognamiglio I, et al. Biomed Res Int. 2014;2014:217365. doi: 10.1155/2014/217365. Epub 2014 Feb 13. Biomed Res Int. 2014. PMID: 24683542 Free PMC article. - Targeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growth.
Leone E, Morelli E, Di Martino MT, Amodio N, Foresta U, Gullà A, Rossi M, Neri A, Giordano A, Munshi NC, Anderson KC, Tagliaferri P, Tassone P. Leone E, et al. Clin Cancer Res. 2013 Apr 15;19(8):2096-106. doi: 10.1158/1078-0432.CCR-12-3325. Epub 2013 Feb 27. Clin Cancer Res. 2013. PMID: 23446999 Free PMC article. - Mir-34: a new weapon against cancer?
Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P, Tassone P, Caraglia M. Misso G, et al. Mol Ther Nucleic Acids. 2014 Sep 23;3(9):e194. doi: 10.1038/mtna.2014.47. Mol Ther Nucleic Acids. 2014. PMID: 25247240 Free PMC article. Review. - MicroRNA theragnostics for the clinical management of multiple myeloma.
Ahmad N, Haider S, Jagannathan S, Anaissie E, Driscoll JJ. Ahmad N, et al. Leukemia. 2014 Apr;28(4):732-8. doi: 10.1038/leu.2013.262. Epub 2013 Sep 12. Leukemia. 2014. PMID: 24714346 Review.
Cited by
- miR‑218 functions as a tumor suppressor gene in cervical cancer.
Liu Z, Mao L, Wang L, Zhang H, Hu X. Liu Z, et al. Mol Med Rep. 2020 Jan;21(1):209-219. doi: 10.3892/mmr.2019.10809. Epub 2019 Nov 11. Mol Med Rep. 2020. PMID: 31746391 Free PMC article. Retracted. - Serum miR-34a-5p and miR-199a-3p as new biomarkers of neonatal sepsis.
Abdelaleem OO, Mohammed SR, El Sayed HS, Hussein SK, Ali DY, Abdelwahed MY, Gaber SN, Hemeda NF, El-Hmid RGA. Abdelaleem OO, et al. PLoS One. 2022 Jan 6;17(1):e0262339. doi: 10.1371/journal.pone.0262339. eCollection 2022. PLoS One. 2022. PMID: 34990478 Free PMC article. - The potential function of microRNAs as biomarkers and therapeutic targets in multiple myeloma.
Zhu B, Ju S, Chu H, Shen X, Zhang Y, Luo X, Cong H. Zhu B, et al. Oncol Lett. 2018 May;15(5):6094-6106. doi: 10.3892/ol.2018.8157. Epub 2018 Mar 2. Oncol Lett. 2018. PMID: 29731841 Free PMC article. Review. - Therapeutic Targeting of MicroRNAs in the Tumor Microenvironment.
Raue R, Frank AC, Syed SN, Brüne B. Raue R, et al. Int J Mol Sci. 2021 Feb 23;22(4):2210. doi: 10.3390/ijms22042210. Int J Mol Sci. 2021. PMID: 33672261 Free PMC article. Review. - Non-coding RNAs and potential therapeutic targeting in cancer.
Toden S, Zumwalt TJ, Goel A. Toden S, et al. Biochim Biophys Acta Rev Cancer. 2021 Jan;1875(1):188491. doi: 10.1016/j.bbcan.2020.188491. Epub 2020 Dec 13. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33316377 Free PMC article. Review.
References
- Tili E, Michaille JJ, Gandhi V, Plunkett W, Sampath D, et al. (2007) miRNAs and their potential for use against cancer and other diseases. Future Oncol 3: 521–537. - PubMed
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, et al. (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433: 769–773. - PubMed
- Bravo V, Rosero S, Ricordi C, Pastori RL (2007) Instability of miRNA and cDNAs derivatives in RNA preparations. Biochem Biophys Res Commun 353: 1052–1055. - PubMed
- De Rosa G, De Stefano D, Galeone A (2010) Oligonucleotide delivery in cancer therapy. Expert Opin Drug Deliv 7: 1263–1278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous