Fluoxetine induces proliferation and inhibits differentiation of hypothalamic neuroprogenitor cells in vitro - PubMed (original) (raw)
Fluoxetine induces proliferation and inhibits differentiation of hypothalamic neuroprogenitor cells in vitro
Lígia Sousa-Ferreira et al. PLoS One. 2014.
Abstract
A significant number of children undergo maternal exposure to antidepressants and they often present low birth weight. Therefore, it is important to understand how selective serotonin reuptake inhibitors (SSRIs) affect the development of the hypothalamus, the key center for metabolism regulation. In this study we investigated the proliferative actions of fluoxetine in fetal hypothalamic neuroprogenitor cells and demonstrate that fluoxetine induces the proliferation of these cells, as shown by increased neurospheres size and number of proliferative cells (Ki-67+ cells). Moreover, fluoxetine inhibits the differentiation of hypothalamic neuroprogenitor cells, as demonstrated by decreased number of mature neurons (Neu-N+ cells) and increased number of undifferentiated cells (SOX-2+ cells). Additionally, fluoxetine-induced proliferation and maintenance of hypothalamic neuroprogenitor cells leads to changes in the mRNA levels of appetite regulator neuropeptides, including Neuropeptide Y (NPY) and Cocaine-and-Amphetamine-Regulated-Transcript (CART). This study provides the first evidence that SSRIs affect the development of hypothalamic neuroprogenitor cells in vitro with consequent alterations on appetite neuropeptides.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
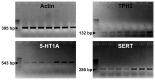
Figure 1. Presence of serotonergic markers in hypothalamic neuroprogenitor cells.
Representative gel electrophoresis with PCR products demonstrating the presence of β-actin (A), neuronal tryptophan hidroxylase (TPH2) (B), serotonin receptor 5-HT1A (C) and serotonin pre-synaptic transport (SERT) (D) in hypothalamic neuroprogenitor cells. PCR was performed in cDNA samples obtained from hypothalamic neurospheres: (1) - P1 control; (2) P1 treated with fluoxetine; (3) - P3 control; (4) - P3 treated with fluoxetine; (5) – differentiated control; (6) – differentiated treated with fluoxetine; and (+) whole adult brain (positive control). P, passage.
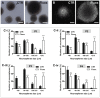
Figure 2. Fluoxetine increases the size of hypothalamic neurospheres.
Hypothalamic neurospheres were incubated with fluoxetine (1 µM) throughout 3 passages. Representative images of P3 hypothalamic neurospheres morphology showed by phase-contrast microscopy (A), and nuclear staining with DAPI (white) (B). (C) Diameter distribution of hypothalamic neurospheres in passages 1 to 4. Mean ± SEM; n = 3/4; Two-way ANOVA; ns, p>0.05; *, p<0.05; **, p<0.01, ***, p<0.001 compared to control. P, passage. Scale bar: 100 µm.
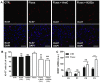
Figure 3. Fluoxetine promotes the proliferation of hypothalamic neuroprogenitor cells.
Hypothalamic neurospheres were incubated with fluoxetine (1 µM) throughout 3 passages. Representative confocal photomicrographs of cell proliferation marker Ki-67 (red) (A). Fluoxetine increases the percentage of Ki-67 positive cells and 24 hours incubation with proliferation inhibitor AraC or Trk receptors inhibitor K252a reverses this effect (B). Fluoxetine upregulates the mRNA levels of neurotrophic factor BDNF (C). DAPI (blue), nuclear staining. Mean ± SEM; n = 4; One-Way ANOVA (B) and Two-Way ANOVA (C); ns, p>0.05; *, p<0.05; ***, p<0.001 compared to control; ##, p<0.01; ###, p<0.001 compared to fluoxetine. Scale bar: 50 µm. P, passage.
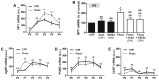
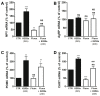
Figure 4. Fluoxetine upregulates the levels of orexigenic neuropeptides NPY and AgRP in hypothalamic neuroprogenitor cells.
Fluoxetine increases the mRNA levels of NPY during P2 and P3 (A). The 24 hours incubation with proliferation inhibitor AraC and Trk receptor inhibitor K252a reverses the increase of NPY mRNA induced by fluoxetine (B). Fluoxetine anticipates the increase of AgRP mRNA in P1 neurospheres (C). Fluoxetine has no effect in the mRNA levels of the anorexigenic neuropeptides: POMC (D) and CART (E). Mean ± SEM; n = 4; Two-Way ANOVA (A, C, D, E) and One-Way ANOVA (B); ns, p>0.05; *, p<0.05; **, p<0.01; ***, p<0.001 compared to control; NS, p>0.05 compared to fluoxetine. P, passage.
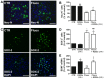
Figure 5. Fluoxetine inhibits the differentiation of hypothalamic neuroprogenitor cells.
Hypothalamic neuroprogenitor cells were differentiated for 18 days, in the presence or absence of fluoxetine (1 µM). Representative confocal photomicrographs for mature neurons marker Neu-N (green) (A). Fluoxetine decreases the percentage of Neu-N positive cells and the incubation with Trk receptors inhibitor K252a (24 h) partially reverses this effect (B). Representative confocal photomicrographs for the progenitor cells marker SOX-2 (green) (C). Fluoxetine increases the percentage of SOX-2 positive cells and the incubation with K252a (24 h) does not change this effect (D). Fluoxetine does not up regulate the levels of neurotrophic factor BDNF but incubation with K252a (24 h) results in a compensatory increase of BDNF mRNA levels (E). DAPI (blue), nuclear staining. Mean ± SEM; n = 4/5; One-Way ANOVA; ns, p>0.05; *, p<0.05; ***, p<0.001 compared to control; NS, p>0.05, #, p<0.05 compared to fluoxetine. Scale bar: 50 µm.
Figure 6. Fluoxetine decreases the levels of abundant neuropeptides (NPY and CART) in hypothalamic differentiated neuroprogenitor cells.
Fluoxetine decreases the mRNA levels of neuropeptides that are abundant in differentiated hypothalamic neuroprogenitor cultures NPY (A) and CART (D), but has no effect in the mRNA of AgRP (B) and POMC (C). Mean ± SEM; n = 5; One-Way ANOVA; ns, p>0.05; *, p<0.05; **, p<0.01; ***, p<0.001 compared to control; NS, p>0.05, #, p<0.05, ###, p<0.001 compared to fluoxetine.
Similar articles
- Proliferative hypothalamic neurospheres express NPY, AGRP, POMC, CART and Orexin-A and differentiate to functional neurons.
Sousa-Ferreira L, Álvaro AR, Aveleira C, Santana M, Brandão I, Kügler S, de Almeida LP, Cavadas C. Sousa-Ferreira L, et al. PLoS One. 2011 May 11;6(5):e19745. doi: 10.1371/journal.pone.0019745. PLoS One. 2011. PMID: 21589937 Free PMC article. - Effects of risperidone treatment on the expression of hypothalamic neuropeptide in appetite regulation in Wistar rats.
Kursungoz C, Ak M, Yanik T. Kursungoz C, et al. Brain Res. 2015 Jan 30;1596:146-55. doi: 10.1016/j.brainres.2014.10.070. Epub 2014 Nov 20. Brain Res. 2015. PMID: 25449893 - Adrenalectomy impairs insulin-induced hypophagia and related hypothalamic changes.
Uchoa ET, Marangon PB, Rorato R, Ruginsk SG, Debarba LK, Antunes-Rodrigues J, Elias LLK. Uchoa ET, et al. J Endocrinol. 2019 Aug;242(2):125-138. doi: 10.1530/JOE-19-0217. J Endocrinol. 2019. PMID: 31189132 - Appetite regulatory neuropeptides are expressed in the sheep hypothalamus before birth.
Mühlhäusler BS, McMillen IC, Rouzaud G, Findlay PA, Marrocco EM, Rhind SM, Adam CL. Mühlhäusler BS, et al. J Neuroendocrinol. 2004 Jun;16(6):502-7. doi: 10.1111/j.1365-2826.2004.01197.x. J Neuroendocrinol. 2004. PMID: 15189324 - FoxO1 Regulates Neuropeptide Y and Pro-opiomelanocortin in the Hypothalamus of Rat Offspring Small for Gestational Age.
Zhang L, Shi Q, Sun Y. Zhang L, et al. Reprod Sci. 2022 Jan;29(1):173-183. doi: 10.1007/s43032-021-00671-7. Epub 2021 Nov 12. Reprod Sci. 2022. PMID: 34767244
Cited by
- Harmine stimulates proliferation of human neural progenitors.
Dakic V, Maciel RM, Drummond H, Nascimento JM, Trindade P, Rehen SK. Dakic V, et al. PeerJ. 2016 Dec 6;4:e2727. doi: 10.7717/peerj.2727. eCollection 2016. PeerJ. 2016. PMID: 27957390 Free PMC article. - Olanzapine add-on treatment promotes neuronal differentiation of neural stem cells compared with fluoxetine alone.
Sun J, Wu D, Xu G, Chen F, Ding X, Xie L, Yu Z, Jin X. Sun J, et al. Biosci Rep. 2022 Oct 28;42(10):BSR20220804. doi: 10.1042/BSR20220804. Biosci Rep. 2022. PMID: 36134560 Free PMC article. - Change of hypothalamic adult neurogenesis in mice by chronic treatment of fluoxetine.
Ohira K. Ohira K. BMC Res Notes. 2022 Feb 16;15(1):60. doi: 10.1186/s13104-022-05954-z. BMC Res Notes. 2022. PMID: 35172883 Free PMC article. - Proliferation rates and gene expression profiles in human lymphoblastoid cell lines from patients with depression characterized in response to antidepressant drug therapy.
Breitfeld J, Scholl C, Steffens M, Brandenburg K, Probst-Schendzielorz K, Efimkina O, Gurwitz D, Ising M, Holsboer F, Lucae S, Stingl JC. Breitfeld J, et al. Transl Psychiatry. 2016 Nov 15;6(11):e950. doi: 10.1038/tp.2016.185. Transl Psychiatry. 2016. PMID: 27845776 Free PMC article. - Fluoxetine Inhibits Canonical Wnt Signaling to Impair Embryoid Body Morphogenesis: Potential Teratogenic Mechanisms of a Commonly Used Antidepressant.
Warkus ELL, Marikawa Y. Warkus ELL, et al. Toxicol Sci. 2018 Oct 1;165(2):372-388. doi: 10.1093/toxsci/kfy143. Toxicol Sci. 2018. PMID: 29893963 Free PMC article.
References
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404: 661–671. - PubMed
- Mercer RE, Chee MJ, Colmers WF (2011) The role of NPY in hypothalamic mediated food intake. Front Neuroendocrinol 32: 398–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by grants from the Portuguese Foundation for Science and Technology, FEDER and COMPETE (PEst-C/SAU/LA0001/2013-2014; PTDC/SAU-FCF/099082/2008; PTDC/BIM-MED/0775/2012; SFRH/BD/30608/2006; SFRH/BPD/73942/2010; SFRH/BPD/78424/2011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous