The prenatal origins of cancer - PubMed (original) (raw)
Review
The prenatal origins of cancer
Glenn M Marshall et al. Nat Rev Cancer. 2014 Apr.
Abstract
The concept that some childhood malignancies arise from postnatally persistent embryonal cells has a long history. Recent research has strengthened the links between driver mutations and embryonal and early postnatal development. This evidence, coupled with much greater detail on the cell of origin and the initial steps in embryonal cancer initiation, has identified important therapeutic targets and provided renewed interest in strategies for the early detection and prevention of childhood cancer.
Figures
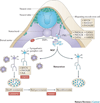
Figure 1. Neural crest development and neuroblastoma
Neuroblast progenitors migrate from the neural crest (nc), around the neural tube (nt) and somites (so) to a region immediately lateral to the notochord (no) and the dorsal aorta (da) under the influence of MYCN and bone morphogenetic proteins (BMPs). At this site the cells undergo specification as the primary sympathetic ganglia (psg) before divergence into neural cells of the mature sympathetic ganglia (sg) or chromaffin cells of the adrenal medulla (am). MYCN is a first hit by virtue of the observations from the tyrosine hydroxylase (Th)-MYCN transgenic mouse model, whereas mutations/alterations in anaplastic lymphoma kinase (ALK) and paired-like homeobox 2 (PHOX2B) are inherited human susceptibility genes. Local access to nerve growth factor (NGF) determines whether the normal sg matures into a terminal ganglion cell or undergoes apoptotic cell death. A relatively common pathologic state is postnatal survival of neuroblast rest disease which requires the cell destined to become malignant to be resistant to trophic factor withdrawal before these persistent rest cells undergo a third change to induce transformation, which presents as a clinical malignancy in early childhood. MASH1, murine achaete-scute homolog 1; HAND2, heart and neural crest derivatives expressed 2; NF-M, neurofilament medium polypeptide.
Figure 2. TMD and ML-DS
Children with Down Syndrome (DS) can develop Transient Myeloproliferative Disease (TMD) at birth which can later transform to Myeloid Leukaemia (ML-DS). The production of megakaryocyte-erythroid progenitors (MEPs) is enhanced in the foetal liver of children with DS and trisomy 21 as a first hit. Some megakaryocyte precursor cells develop a second hit mutation in GATA1 resulting in a mutant, truncated protein, GATA1s. All children with TMD and ML-DS exhibit GATA1s and more than two copies of chromosome 21. TMD resolves clinically in almost all patients to later present as ML-DS in 25% of TMD cases. Thus, some TMD cells at birth must survive through unique death resistance mechanisms and may undergo further changes which lead to genomic instability and clinical presentation as ML-DS.
Figure 3. The development of B-ALL
The presence of specific prenatal oncogenic fusion proteins in haematopoietic stem cells (HSCs) or B progenitor cells combines with aberrant expression of transcription factors necessary for normal B cell development and maturation during the genesis of acute lymphoblastic leukaemia (B-ALL). Foetal haematopoiesis begins in the haemogenic endothelium formed from the yolk sac and aorto-gonadal-mesonephros (AGM), then localizes to the foetal liver, to finally reside in the bone marrow from the perinatal period. Several fusion genes (ETV6-RUNX1, MLL-ENL, BCR-ABL) present as clonal chromosome rearrangements at the point of diagnosis of B-ALL, represent the first hit as they have also been identified in blood samples at birth in children who later develop B-ALL. Since the incidence of the ETV6-RUNX1 fusion gene is higher than the clinical incidence of B-ALL, some B-ALL rest cells must survive the perinatal period to undergo a third hit that leads to clinical presentation.
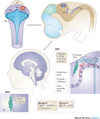
Figure 4. Cerebellar development and embryonal origin of medulloblastoma
The upper (anterior) rhombic lip (URL) is a germinal zone of proliferating granule cell precursors (GCPs) that migrate rostrally to form the external granular layer (EGL). A second germinal centre is the ventricular zone (VZ), which gives rise to Purkinje cells and several other types of cerebellar interneurons. Sonic hedgehog (SHH) tumours may arise from persistent cells of the EGL that have not migrated to the internal granular layer (IGL), while Wnt tumours may originate from persistent cells of the ventricular zone in the brainstem (BS). Group 3 and 4 medulloblastomas likely arise from neural stem cells of the hindbrain.
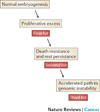
Figure 5. A model of embryonal tumorigenesis
We propose that each of the four embryonal malignancies with evidence of a prenatal origin (neuroblastoma, ML-DS, B-ALL, medulloblastoma) share common features: (i) a prenatal proliferative excess in the tissue of origin; (ii) a cell intrinsic mechanism for surviving the hostile early postnatal environment; (iii) an accelerated pathway toward genomic instability.
Similar articles
- Development and cancer of the cerebellum.
Hatten ME, Roussel MF. Hatten ME, et al. Trends Neurosci. 2011 Mar;34(3):134-42. doi: 10.1016/j.tins.2011.01.002. Trends Neurosci. 2011. PMID: 21315459 Free PMC article. Review. - Medulloblastoma as a first presentation of fanconi anemia.
Tischkowitz MD, Chisholm J, Gaze M, Michalski A, Rosser EM. Tischkowitz MD, et al. J Pediatr Hematol Oncol. 2004 Jan;26(1):52-5. doi: 10.1097/00043426-200401000-00016. J Pediatr Hematol Oncol. 2004. PMID: 14707715 Review. - Direct effects of Bmi1 on p53 protein stability inactivates oncoprotein stress responses in embryonal cancer precursor cells at tumor initiation.
Calao M, Sekyere EO, Cui HJ, Cheung BB, Thomas WD, Keating J, Chen JB, Raif A, Jankowski K, Davies NP, Bekkum MV, Chen B, Tan O, Ellis T, Norris MD, Haber M, Kim ES, Shohet JM, Trahair TN, Liu T, Wainwright BJ, Ding HF, Marshall GM. Calao M, et al. Oncogene. 2013 Aug 1;32(31):3616-26. doi: 10.1038/onc.2012.368. Epub 2012 Aug 20. Oncogene. 2013. PMID: 22907436 - Neoplasia in childhood--25 years of progress.
Mott MG. Mott MG. Ann Oncol. 1995;6 Suppl 1:3-8; discussion 8-9. doi: 10.1093/annonc/6.suppl_1.s3. Ann Oncol. 1995. PMID: 8695541 Review. - The "neuro" of neuroblastoma: Neuroblastoma as a neurodevelopmental disorder.
Ratner N, Brodeur GM, Dale RC, Schor NF. Ratner N, et al. Ann Neurol. 2016 Jul;80(1):13-23. doi: 10.1002/ana.24659. Epub 2016 Apr 30. Ann Neurol. 2016. PMID: 27043043 Free PMC article. Review.
Cited by
- Down syndrome-associated leukaemias: current evidence and challenges.
Mason NR, Cahill H, Diamond Y, McCleary K, Kotecha RS, Marshall GM, Mateos MK. Mason NR, et al. Ther Adv Hematol. 2024 Jul 23;15:20406207241257901. doi: 10.1177/20406207241257901. eCollection 2024. Ther Adv Hematol. 2024. PMID: 39050114 Free PMC article. Review. - Development and validation of a novel stemness-related prognostic model for neuroblastoma using integrated machine learning and bioinformatics analyses.
Xia Y, Wang C, Li X, Gao M, Hogg HDJ, Tunthanathip T, Hulsen T, Tian X, Zhao Q. Xia Y, et al. Transl Pediatr. 2024 Jan 29;13(1):91-109. doi: 10.21037/tp-23-582. Epub 2024 Jan 12. Transl Pediatr. 2024. PMID: 38323183 Free PMC article. - TWIST1 expression is associated with high-risk neuroblastoma and promotes primary and metastatic tumor growth.
Sepporta MV, Praz V, Balmas Bourloud K, Joseph JM, Jauquier N, Riggi N, Nardou-Auderset K, Petit A, Scoazec JY, Sartelet H, Renella R, Mühlethaler-Mottet A. Sepporta MV, et al. Commun Biol. 2022 Jan 12;5(1):42. doi: 10.1038/s42003-021-02958-6. Commun Biol. 2022. PMID: 35022561 Free PMC article. - Future Match Making: When Pediatric Oncology Meets Organoid Technology.
Barbet V, Broutier L. Barbet V, et al. Front Cell Dev Biol. 2021 Jul 13;9:674219. doi: 10.3389/fcell.2021.674219. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34327198 Free PMC article. Review. - Childhood cancer in children with congenital anomalies in Oklahoma, 1997 to 2009.
Janitz AE, Neas BR, Campbell JE, Pate AE, Stoner JA, Magzamen SL, Peck JD. Janitz AE, et al. Birth Defects Res A Clin Mol Teratol. 2016 Jul;106(7):633-42. doi: 10.1002/bdra.23494. Epub 2016 Mar 4. Birth Defects Res A Clin Mol Teratol. 2016. PMID: 26945683 Free PMC article.
References
- Valent P, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767–775. - PubMed
- Gruhn B, et al. Prenatal origin of childhood acute lymphoblastic leukemia, association with birth weight and hyperdiploidy. Leukemia. 2008;22:1692–1697. - PubMed
- Pine SR, et al. Incidence and clinical implications of GATA1 mutations in newborns with Down syndrome. Blood. 2007;110:2128–2131. - PubMed
- Beckwith JB. Precursor lesions of Wilms tumor: clinical and biological implications. Med Pediatr Oncol. 1993;21:158–168. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
- U54 CA163155/CA/NCI NIH HHS/United States
- R01 CA102321/CA/NCI NIH HHS/United States
- U01 CA176287/CA/NCI NIH HHS/United States
- R01 CA133091/CA/NCI NIH HHS/United States
- F30 CA174154/CA/NCI NIH HHS/United States
- R01 CA159859/CA/NCI NIH HHS/United States
- R01 CA148699/CA/NCI NIH HHS/United States
- T32 GM064337/GM/NIGMS NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- P01 CA081403/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases