Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial - PubMed (original) (raw)
Clinical Trial
. 2014 Jun;25(6):1128-36.
doi: 10.1093/annonc/mdu118. Epub 2014 Mar 11.
S Litière 2, M Piccart 3, G MacGrogan 4, P Fumoleau 5, E Brain 6, T Petit 7, P Rouanet 8, J Jassem 9, C Moldovan 10, A Bodmer 11, K Zaman 12, T Cufer 13, M Campone 14, E Luporsi 15, P Malmström 16, G Werutsky 2, J Bogaerts 2, J Bergh 17, D A Cameron 18; EORTC 10994/BIG 1-00 Study investigators
Affiliations
- PMID: 24618153
- PMCID: PMC4037859
- DOI: 10.1093/annonc/mdu118
Clinical Trial
Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial
H Bonnefoi et al. Ann Oncol. 2014 Jun.
Abstract
Background: Pathological complete response (pCR) following chemotherapy is strongly associated with both breast cancer subtype and long-term survival. Within a phase III neoadjuvant chemotherapy trial, we sought to determine whether the prognostic implications of pCR, TP53 status and treatment arm (taxane versus non-taxane) differed between intrinsic subtypes.
Patients and methods: Patients were randomized to receive either six cycles of anthracycline-based chemotherapy or three cycles of docetaxel then three cycles of eprirubicin/docetaxel (T-ET). pCR was defined as no evidence of residual invasive cancer (or very few scattered tumour cells) in primary tumour and lymph nodes. We used a simplified intrinsic subtypes classification, as suggested by the 2011 St Gallen consensus. Interactions between pCR, TP53 status, treatment arm and intrinsic subtype on event-free survival (EFS), distant metastasis-free survival (DMFS) and overall survival (OS) were studied using a landmark and a two-step approach multivariate analyses.
Results: Sufficient data for pCR analyses were available in 1212 (65%) of 1856 patients randomized. pCR occurred in 222 of 1212 (18%) patients: 37 of 496 (7.5%) luminal A, 22 of 147 (15%) luminal B/HER2 negative, 51 of 230 (22%) luminal B/HER2 positive, 43 of 118 (36%) HER2 positive/non-luminal, 69 of 221(31%) triple negative (TN). The prognostic effect of pCR on EFS did not differ between subtypes and was an independent predictor for better EFS [hazard ratio (HR) = 0.40, P < 0.001 in favour of pCR], DMFS (HR = 0.32, P < 0.001) and OS (HR = 0.32, P < 0.001). Chemotherapy arm was an independent predictor only for EFS (HR = 0.73, P = 0.004 in favour of T-ET). The interaction between TP53, intrinsic subtypes and survival outcomes only approached statistical significance for EFS (P = 0.1).
Conclusions: pCR is an independent predictor of favourable clinical outcomes in all molecular subtypes in a two-step multivariate analysis.
Clinicaltrialsgov: EORTC 10994/BIG 1-00 Trial registration number NCT00017095.
Keywords: TP53; breast cancer; landmark analysis; neoadjuvant chemotherapy; pathological complete response.
© The Author 2014. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
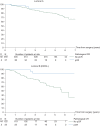
Figure 1.
Effect of pCR on EFS by intrinsic subtype (N = 1212 eligible and assessable patients). pCR, pathological complete response; EFS, event-free survival.
Figure 1.
Effect of pCR on EFS by intrinsic subtype (N = 1212 eligible and assessable patients). pCR, pathological complete response; EFS, event-free survival.
Figure 1.
Effect of pCR on EFS by intrinsic subtype (N = 1212 eligible and assessable patients). pCR, pathological complete response; EFS, event-free survival.
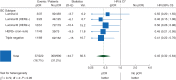
Figure 2.
Effect of pCR on EFS by intrinsic subtype: univariate Cox regression models (N = 1212 eligible and assessable patients). pCR, pathological complete response; EFS, event-free survival; HR, hazard ratio; CI, confidence interval; HER2, human epidermal growth factor 2; df, degrees of freedom.
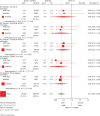
Figure 3.
Effect of pCR on event-free survival by intrinsic subtype and TP53 status: univariate Cox regression models (N = 1212 eligible and assessable patients)a. pCR, pathological complete response; HR, hazard ratio; CI, confidence interval; HER2, human epidermal growth factor 2; df, degrees of freedom. aTwo hundred and thirty-seven patients with p53 status missing were not considered in this graph.
References
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. - PubMed
- Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous