The nuclear pore complex function of Sec13 protein is required for cell survival during retinal development - PubMed (original) (raw)
The nuclear pore complex function of Sec13 protein is required for cell survival during retinal development
Xubo Niu et al. J Biol Chem. 2014.
Abstract
Sec13 is a dual function protein, being a core component of both the COPII coat, which mediates protein trafficking from the endoplasmic reticulum to the Golgi apparatus, and the nuclear pore complex (NPC), which facilitates nucleo-cytoplasmic traffic. Here, we present a genetic model to differentiate the roles of these two functions of Sec13 in vivo. We report that sec13(sq198) mutant embryos develop small eyes that exhibit disrupted retinal lamination and that the mutant retina contains an excessive number of apoptotic cells. Surprisingly, we found that loss of COPII function by oligonucleotide-mediated gene knockdown of sec31a and sec31b or brefeldin A treatment did not disrupt retinal lamination, although it did result in digestive organ defects similar to those seen in sec13(sq198), suggesting that the digestive organ defects observed in sec13(sq198) are due to loss of COPII function, whereas the retinal lamination defects are due to loss of the NPC function. We showed that the retinal cells of sec13(sq198) failed to form proper nuclear pores, leading to a nuclear accumulation of total mRNA and abnormal activation of the p53-dependent apoptosis pathway, causing the retinal defect in sec13(sq198). Furthermore, we found that a mutant lacking Nup107, a key NPC-specific component, phenocopied the retinal lamination phenotype as observed in sec13(sq198). Our results demonstrate a requirement for the nuclear pore function of Sec13 in development of the retina and provide the first genetic evidence to differentiate the contributions of the NPC and the COPII functions of Sec13 during organogenesis.
Keywords: Cell Biology; Endoplasmic Reticulum (ER); Molecular Genetics; Nuclear Pore Complexes; Retina Development; Sec13; Sec31; Zebrafish; p53.
Figures
FIGURE 1.
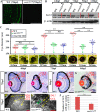
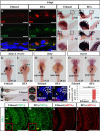
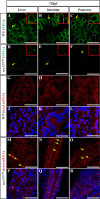
Retinal lamination is disrupted in the sec13sq198 mutant. A, immunostaining of Sec13 protein in the WT and sec13sq198 mutant retina at 72 hpf. B, Sec13 is a maternal deposited protein. Western blot analysis of Sec13 protein in WT and sec13sq198 mutant from 24 to 120 hpf is shown. Value for Sec13 protein in WT at 24 hpf is set as 1. C, measurements of eye diameter in WT, sec13sq198 mutant, and sec31a/sec31b double morphant at 48, 72, and 96 hpf (n = 18). D–G, H&E staining analysis of WT (D and F) and mutant (E and G) retina at 72 and 96 hpf. WT embryos at 72 and 96 hpf developed normally laminated retinas consisting of a differentiated GCL, an INL, and an ONL (indicated by the red arrows). However, retinal lamination in sec13sq198 mutant embryos was disrupted (indicated by the red arrows), and retinal pigment epithelium was clearly expanded in width (shown by the red arrowheads). H and I, TEM analysis of the WT and sec13sq198 mutant retina at 72 hpf showed that compared with well developed WT retina (H), the sec13sq198 mutant embryos developed disrupted and disorganized retina containing many apoptotic cells (shown by the green arrows) (I). J, average number of GCL cells per section/per embryo in each genotype is shown (n = 3, four sections from each embryo were used for counting the GCL cells). L, lens. Scale bar, 50 μm (A and D–G), 150 μm (C), and 20 μm (H and I). NS, no significance. *, p < 0.05; **, p < 0.01.
FIGURE 2.
Differentiation of the retinal cells is not affected in the sec13sq198 mutant. A, WISH analysis of early eye markers otx2 and rx1 in the WT and sec13sq198 mutant embryos at 36 hpf. B–M, in situ hybridization analysis of different retinal cell types in the WT and sec13sq198 mutant at 72 hpf. Examination of each cell type in the WT (B, D, F, H, J, and L) and sec13sq198 mutant (C, E, G, I, K, and M) retina are shown. Ganglion cells were detected with the following: brn3b probe (B and C); amacrine cells with an anti-serotonin antibody (D and E); bipolar cells with a vsx1 probe (F and G); rod photoreceptors with a rod opsin probe (H and I); blue cones with a blue opsin probe (J and K); and red cones with a red opsin probe (L and M). N, bright field images (top panel) of the retina of the WT, sec13sq198 mutant, sec13 morphant, and sec13sq198 mutant embryos injected with sec13 mRNA at 72 hpf and WISH analysis (lower panel) of rod opsin expression in the retina of the WT, sec13sq198 mutant, sec13 morphant, and sec13sq198 mutant embryos injected with sec13 mRNA at 72 hpf. L, lens. Scale bar, 50 μm (B–G) and 150 μm (N).
FIGURE 3.
ER lumen in sec13sq198 mutant retinal cells. A–F, TEM analysis of the ER structure in a GCL cell (A and D), INL cell (B and E), and ONL cell (C and F) in WT (A–C) and sec13sq198 mutants (D–F) at 72 hpf. ER lumens are indicated with yellow arrows. Scale bar, 0.5 μm.
FIGURE 4.
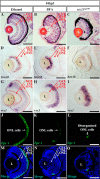
Loss-of-function of COPII does not disrupt retinal lamination. A, expression of sec31b in the embryos injected with the standard control morpholino (st MO) or two sec31b splicing morpholinos (sec31b MO1 and sec31b MO2) at 12 and 24 hpf were determined by qPCR with the expression of elf1a as the normalization control. B–D, WISH analysis of digestive organs in the control morphant (st MO), sec31b morphant, and sec31a/sec31b double morphant at 72 hpf. In each case, the number of total embryos examined (as denominator) and the number of embryos exhibiting the displayed phenotype (as numerator) are shown on the bottom right. E and F, H&E staining of the sections of eyes in the control (E) and sec31a/sec31b double (F) morphant. G–L, ganglion cells were detected with a brn3b probe (G and H), bipolar cells with a vsx1 probe (I and J), and green/red double cones with an anti-Zpr-1 antibody (K and L). M, comparison of the number of apoptotic cells in the control and sec31a/sec31b double morphant at 72 hpf (n = 3, four sections from each embryo were used for counting the apoptotic cells). N–R, histological analysis of the retinal structure in Sec23b morphant at 120 hpf and in Sec24c morphant at 144 hpf. L, lens. Scale bar, 50 μm (E–L).
FIGURE 5.
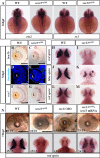
BFA treatment destroys the COPII function and impedes digestive organ development. A–F, double immunostaining of PDI (ER marker) and collagen II (Col2a1, secretory protein) in ethanol-treated (A–C) and BFA-treated (D–F) embryos at 84 hpf. A and D, immunostaining of PDI (shown in red). B and E, immunostaining of Col2a1 (in green). C and F, merge of PDI and Col2a1 staining. G–J, WISH analysis of chop and bip in ethanol-treated (G and I) and BFA-treated (H and J) embryos at 84 hpf. K–P, WISH analysis using lfabp (for the liver) plus insulin (for the endocrine pancreas) (K and L), ifabp (for the intestine) (M and N), and trypsin (for the exocrine pancreas) (O and P) probes for the analysis of digestive organs in ethanol-treated (K, M, and O) and BFA-treated (L, N, and P) embryos at 84 hpf. In each case, the number of total embryos examined (as denominator) and the number of embryos exhibiting the displayed phenotype (as numerator) are shown on the bottom right. Q and R, bright field images of ethanol-treated (Q) and BFA-treated (R) embryos at 84 hpf. Higher magnification of the eye image is shown on the right correspondingly. S–U, representative image of TUNEL analysis of apoptosis in ethanol-treated (S) and BFA-treated (T) retina at 84 hpf. U, average number of apoptotic cells in these samples were shown (n = 3, four sections from each embryo were used for counting the apoptotic cells). V and W, immunostaining of nuclear pores in ethanol-treated (V) and BFA-treated (W) embryos at 84 hpf. X and Y, mRNA export assay in ethanol-treated (X) and BFA-treated (Y) retinas at 84 hpf. L, lens. Scale bar, 10 μm (A–F), 300 μm (Q and R), 150 μm (inset in Q and R), and 50 μm (S, T, and V–Y). **, p < 0.01.
FIGURE 6.
BFA treatment does not disrupt retinal lamination. A–C, H&E staining analysis of the retina in ethanol-treated (A), BFA-treated (B), and sec13sq198 mutant (C) embryos at 84 hpf. D–O, ganglion cells were detected with a brn3b probe (D–F), bipolar cells with a vsx1 probe (G–I), and green/red double cones with an anti-Zpr-1 antibody (J–O). L, lens. Scale bar, 50 μm.
FIGURE 7.
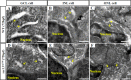
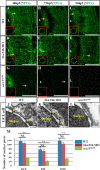
Formation of nuclear pores is impaired in the sec13sq198 mutant retina. A–I, immunostaining of nuclear pores in WT (A–C), sec131a/sec31b double morphant (D–F), and sec13sq198 mutant (G–I) retina with Mab414 antibody. The nuclear pores in both WT (A–C) and the sec31a/sec31b double morphant (D–F) retina were well formed (shown by the yellow arrows and insets), whereas formation of the nuclear pores in the sec13sq198 mutant retina gradually failed (G–I) (shown by the yellow arrows and insets). J–L, TEM analysis of the nuclear pores in the GCL cells in WT (J), sec31a/sec31b double morphant (K), and sec13sq198 mutant (L) retina at 72 hpf. Note that both the WT (J) and the sec31a/sec31b double morphant (K) retinal cells displayed characteristic nuclear pores (indicated by the yellow arrows). In contrast, the mutant retina was defective in the formation of nuclear pores in cells from all three layers. M, comparison of the number of nuclear pores in the GCL, INL, and ONL in the WT, sec31a/sec31b double morphants, and sec13sq198 mutant retina at 72 hpf. Three ultrathin sections from two embryos for each genotype were used to count the number of nuclear pores in the GCL, INL, and ONL, respectively. For each ultrathin section, six cells in each retinal layer were selected for counting, and the total number of nuclear pores from 18 cells in each retinal layer is shown here. L, lens. Scale bar, 50 μm (A–I) and 1 μm (J–L). NS, no significance. **, p < 0.01.
FIGURE 8.
Total polyadenylated mRNAs accumulate in retinal cell nuclei in the sec13sq198 mutant. A–R, mRNA export assay as marked by Cy3-labeled oligo(dT)50 in WT (A–C and J–L), sec31a/sec31b double morphant (D–F and M–O), and sec13sq198 mutant (G–I and P–R) retinas at 48 and 72 hpf. In both WT (A–C and J–L) and sec31a/sec31b double morphant (D–F and M–O) retinal cells, mRNA was evenly distributed throughout the cytoplasm. However, in the sec13sq198 mutant retinal cells (G–I and P–R), export was blocked, and mRNA accumulated in the nuclei (shown by the yellow arrows). Scale bar, 50 μm.
FIGURE 9.
sec13sq198 mutant digestive organs are also defective in the formation of nuclear pores and mRNA export. A–F, immunostaining of nuclear pores with a mouse monoclonal Mab414 antibody in the WT (A–C) and sec13sq198 mutant (D–F) liver, intestine, and pancreatic cells at 72 hpf. G–R, mRNA export assay in WT (G–L) and sec13sq198 mutant (M–R) liver, intestine, and pancreatic cells at 72 hpf. The nuclei were stained with DAPI. Scale bar, 15 μm (A–F) and 25 μm (G–R).
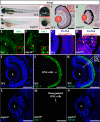
FIGURE 10.
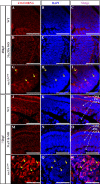
Loss-of-function of the NPC component Nup107 phenocopies the sec13sq198 retinal phenotype. A and B, WT embryo (A) and a nup107tsu068Gt mutant (nup107) embryo (B) at 84 hpf. Higher magnification of the eye image is shown on the right correspondingly. C and D, H&E staining analysis of the retina in the WT (C) and nup107tsu068Gt mutant (D) at 84 hpf. E and F, immunostaining of the NPCs in nuclear pores in WT (E) and nup107tsu068Gt mutant (F) at 84 hpf. G and H, mRNA export assay in the WT (G) and nup107tsu068Gt mutant (H) at 84 hpf. I–N, immunostaining of Zpr-1 in the WT (I–K) and nup107tsu068Gt mutant (L–N) retina at 84 hpf. L, lens. Scale bar, 300 μm (A and B), 150 μm (insets in A and B), and 50 μm (C, D, and I–N).
FIGURE 11.
Failure in the formation of nuclear pores triggers cell apoptosis, causing the disruption of retinal lamination in the sec13sq198 mutant. A, average number of PH3 positive cells per section/embryo in WT and sec13sq198 mutant retina is shown (n = 3, four sections from each embryo were used for counting the PH3-ositive cells). B and C, representative image of PH3 staining in the WT (B) and sec13sq198 mutant (C) retina at 48 hpf. D, average number of apoptotic cells in the WT and sec13sq198 mutant retina at 30, 48, and 72 hpf (n = 3, four sections from each embryo were used for counting the apoptotic cells). E and F, representative image of the TUNEL assays of apoptotic cells in WT (E) and sec13sq198 mutant (F) retina at 72 hpf. G–J, drawings represent the retinal structure by highlighting the cells in the GCL, INL, and ONL in the WT, BFA-treated (representing COPII-dysfunction), sec13sq198 mutant, and nup107tsu068Gt mutant (representing NPC dysfunction) at 84 hpf. K–N, drawings based on TUNEL analysis of apoptotic cells in different genotypes (entire retina). L, lens. Scale bar, 50 μm. NS, no significance. **, p < 0.01.
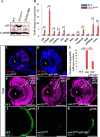
FIGURE 12.
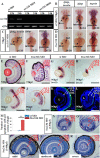
Knockdown of p53 partially restores the sec13sq198 mutant retina lesion. A, Western blot of p53 protein in the WT, sec31a/sec31b double morphant, and sec13sq198 mutant retina. Value for p53 protein in WT is set as 1. B, qPCR analysis of the expression of genes of the p53 and apoptosis pathway in the WT and sec13sq198 mutant retina. The relative expression level for each gene was expressed as the fold change in expression after normalization against elf1a. The p value was obtained by performing the two-tailed unpaired t test. C and D, representative image of the TUNEL assays of apoptotic cells in sec13sq198 mutant (C) and p53-ATG morpholino (p53 MO)-injected sec13sq198 mutant (D) retina at 72 hpf. E, average number of apoptotic cells in the WT, sec13sq198 mutant, and p53 MO-injected sec13sq198 mutant retina at 72 hpf (n = 3, four sections from each embryo were used for counting the apoptotic cells in each case). F–H, overview of the retina in WT (F), sec13sq198 mutant (G), and p53 MO-injected sec13sq198 mutant (H) embryos. I–K, immunostaining of Zpr-1 in WT (I), sec13sq198 mutant (J), and p53 MO-injected sec13sq198 mutant (K) retinas. Clearly, a knockdown of p53 partially restored the layered structure (H) (outlined with white lines) and ONL (K) in the sec13sq198 mutant retina. L, lens. Scale bar, 50 μm (C, D, and F–K). **, p < 0.01.
Similar articles
- Sec13 safeguards the integrity of the endoplasmic reticulum and organogenesis of the digestive system in zebrafish.
Niu X, Gao C, Jan Lo L, Luo Y, Meng C, Hong J, Hong W, Peng J. Niu X, et al. Dev Biol. 2012 Jul 15;367(2):197-207. doi: 10.1016/j.ydbio.2012.05.004. Epub 2012 May 15. Dev Biol. 2012. PMID: 22609279 - Early stages of retinal development depend on Sec13 function.
Schmidt K, Cavodeassi F, Feng Y, Stephens DJ. Schmidt K, et al. Biol Open. 2013 Mar 15;2(3):256-66. doi: 10.1242/bio.20133251. Epub 2013 Jan 10. Biol Open. 2013. PMID: 23519012 Free PMC article. - Loss of zygotic NUP107 protein causes missing of pharyngeal skeleton and other tissue defects with impaired nuclear pore function in zebrafish embryos.
Zheng X, Yang S, Han Y, Zhao X, Zhao L, Tian T, Tong J, Xu P, Xiong C, Meng A. Zheng X, et al. J Biol Chem. 2012 Nov 2;287(45):38254-64. doi: 10.1074/jbc.M112.408997. Epub 2012 Sep 10. J Biol Chem. 2012. PMID: 22965233 Free PMC article. - Epithelial organization and cyst lumen expansion require efficient Sec13-Sec31-driven secretion.
Townley AK, Schmidt K, Hodgson L, Stephens DJ. Townley AK, et al. J Cell Sci. 2012 Feb 1;125(Pt 3):673-84. doi: 10.1242/jcs.091355. Epub 2012 Feb 13. J Cell Sci. 2012. PMID: 22331354 Free PMC article. - The highly conserved COPII coat complex sorts cargo from the endoplasmic reticulum and targets it to the golgi.
Lord C, Ferro-Novick S, Miller EA. Lord C, et al. Cold Spring Harb Perspect Biol. 2013 Feb 1;5(2):a013367. doi: 10.1101/cshperspect.a013367. Cold Spring Harb Perspect Biol. 2013. PMID: 23378591 Free PMC article. Review.
Cited by
- Moderate Nucleoporin 133 deficiency leads to glomerular damage in zebrafish.
Cianciolo Cosentino C, Berto A, Pelletier S, Hari M, Loffing J, Neuhauss SCF, Doye V. Cianciolo Cosentino C, et al. Sci Rep. 2019 Mar 18;9(1):4750. doi: 10.1038/s41598-019-41202-4. Sci Rep. 2019. PMID: 30894603 Free PMC article. - Role of Nucleoporins and Transport Receptors in Cell Differentiation.
Khan AU, Qu R, Ouyang J, Dai J. Khan AU, et al. Front Physiol. 2020 Apr 3;11:239. doi: 10.3389/fphys.2020.00239. eCollection 2020. Front Physiol. 2020. PMID: 32308628 Free PMC article. Review. - Non-canonical role of cancer-associated mutant SEC23B in the ribosome biogenesis pathway.
Yehia L, Jindal S, Komar AA, Eng C. Yehia L, et al. Hum Mol Genet. 2018 Sep 15;27(18):3154-3164. doi: 10.1093/hmg/ddy226. Hum Mol Genet. 2018. PMID: 29893852 Free PMC article. - Copper induce zebrafish retinal developmental defects via triggering stresses and apoptosis.
Zhao G, Sun H, Zhang T, Liu JX. Zhao G, et al. Cell Commun Signal. 2020 Mar 14;18(1):45. doi: 10.1186/s12964-020-00548-3. Cell Commun Signal. 2020. PMID: 32169084 Free PMC article. - Nucleoporin genes in human diseases.
Nofrini V, Di Giacomo D, Mecucci C. Nofrini V, et al. Eur J Hum Genet. 2016 Oct;24(10):1388-95. doi: 10.1038/ejhg.2016.25. Epub 2016 Apr 13. Eur J Hum Genet. 2016. PMID: 27071718 Free PMC article. Review.
References
- Antonny B., Schekman R. (2001) ER export: public transportation by the COPII coach. Curr. Opin. Cell Biol. 13, 438–443 - PubMed
- Stagg S. M., Gürkan C., Fowler D. M., LaPointe P., Foss T. R., Potter C. S., Carragher B., Balch W. E. (2006) Structure of the Sec13/31 COPII coat cage. Nature 439, 234–238 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous