Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma - PubMed (original) (raw)
Clinical Trial
. 2014 May 1;20(9):2457-65.
doi: 10.1158/1078-0432.CCR-13-3017. Epub 2014 Mar 14.
Begoña Comin-Anduix, Bartosz Chmielowski, Richard C Koya, Zhongqi Wu, Martin Auerbach, Charles Ng, Earl Avramis, Elizabeth Seja, Arturo Villanueva, Tara A McCannel, Akira Ishiyama, Johannes Czernin, Caius G Radu, Xiaoyan Wang, David W Gjertson, Alistair J Cochran, Kenneth Cornetta, Deborah J L Wong, Paula Kaplan-Lefko, Omid Hamid, Wolfram Samlowski, Peter A Cohen, Gregory A Daniels, Bijay Mukherji, Lili Yang, Jerome A Zack, Donald B Kohn, James R Heath, John A Glaspy, Owen N Witte, David Baltimore, James S Economou, Antoni Ribas
Affiliations
- PMID: 24634374
- PMCID: PMC4070853
- DOI: 10.1158/1078-0432.CCR-13-3017
Clinical Trial
Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma
Thinle Chodon et al. Clin Cancer Res. 2014.
Abstract
Purpose: It has been demonstrated that large numbers of tumor-specific T cells for adoptive cell transfer (ACT) can be manufactured by retroviral genetic engineering of autologous peripheral blood lymphocytes and expanding them over several weeks. In mouse models, this therapy is optimized when administered with dendritic cell (DC) vaccination. We developed a short 1-week manufacture protocol to determine the feasibility, safety, and antitumor efficacy of this double cell therapy.
Experimental design: A clinical trial (NCT00910650) adoptively transferring MART-1 T-cell receptor (TCR) transgenic lymphocytes together with MART-1 peptide-pulsed DC vaccination in HLA-A2.1 patients with metastatic melanoma. Autologous TCR transgenic cells were manufactured in 6 to 7 days using retroviral vector gene transfer, and reinfused with (n = 10) or without (n = 3) prior cryopreservation.
Results: A total of 14 patients with metastatic melanoma were enrolled and 9 of 13 treated patients (69%) showed evidence of tumor regression. Peripheral blood reconstitution with MART-1-specific T cells peaked within 2 weeks of ACT, indicating rapid in vivo expansion. Administration of freshly manufactured TCR transgenic T cells resulted in a higher persistence of MART-1-specific T cells in the blood as compared with cryopreserved. Evidence that DC vaccination could cause further in vivo expansion was only observed with ACT using noncryopreserved T cells.
Conclusion: Double cell therapy with ACT of TCR-engineered T cells with a very short ex vivo manipulation and DC vaccines is feasible and results in antitumor activity, but improvements are needed to maintain tumor responses.
©2014 AACR.
Figures
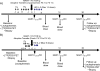
Figure 1. F5 study outline
A) Schedule of events for patients F5-1 to F5-11 who received cryopreserved TCR transgenic cells under amendments 1-7. B) Schedule of events for patients F5-12 to F5-14 who received freshly manufactured TCR transgenic cells after amendment 8.
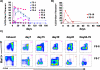
Figure 2. Post-infusion peripheral blood levels of MART-1 TCR transgenic cells at various time points in patients receiving cryopreserved transgenic cells
A) F5-1 to F5-9 receiving up to 109 cryopreserved transgenic cells. B) F5-10 and F5-11 receiving up to 1010 cryopreserved transgenic cells. C) Representative dot plots of MART-1 MHC tetramer analysis of infused cells and post-infusion peripheral blood PBMC in F5-7 and F5-8.
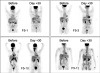
Figure 3. Pre- and post-treatment day 30 PET scans indicating initial antitumor activity
Representative scans of F5-1 and F5-3 receiving up to 109 cryopreserved transgenic cells, F5-10 and F5-11 receiving up to 1010 cryopreserved transgenic cells.
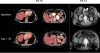
Figure 4. Pre- and post-treatment day 35 PET/CT (F5-10) and CT (F5-13) showing evidence of initial antitumor activity
Representative scans of F5-10 receiving up to 1010 cryopreserved transgenic cells and F5-13 receiving up to 1010 freshly harvested transgenic cells.
Figure 5. Post-infusion peripheral blood levels of MART-1 TCR transgenic cells at various time points in patients receiving freshly harvested transgenic cells
MART-1 tetramer positive CD4+ and CD8+ levels in F5-12, F5-13 and F5-14 receiving up to 1010 freshly harvested transgenic cells.
Similar articles
- Randomized phase II trial of lymphodepletion plus adoptive cell transfer of tumor-infiltrating lymphocytes, with or without dendritic cell vaccination, in patients with metastatic melanoma.
Saberian C, Amaria RN, Najjar AM, Radvanyi LG, Haymaker CL, Forget MA, Bassett RL, Faria SC, Glitza IC, Alvarez E, Parshottam S, Prieto V, Lizée G, Wong MK, McQuade JL, Diab A, Yee C, Tawbi HA, Patel S, Shpall EJ, Davies MA, Hwu P, Bernatchez C. Saberian C, et al. J Immunother Cancer. 2021 May;9(5):e002449. doi: 10.1136/jitc-2021-002449. J Immunother Cancer. 2021. PMID: 34021033 Free PMC article. Clinical Trial. - A Pilot Trial of the Combination of Transgenic NY-ESO-1-reactive Adoptive Cellular Therapy with Dendritic Cell Vaccination with or without Ipilimumab.
Nowicki TS, Berent-Maoz B, Cheung-Lau G, Huang RR, Wang X, Tsoi J, Kaplan-Lefko P, Cabrera P, Tran J, Pang J, Macabali M, Garcilazo IP, Carretero IB, Kalbasi A, Cochran AJ, Grasso CS, Hu-Lieskovan S, Chmielowski B, Comin-Anduix B, Singh A, Ribas A. Nowicki TS, et al. Clin Cancer Res. 2019 Apr 1;25(7):2096-2108. doi: 10.1158/1078-0432.CCR-18-3496. Epub 2018 Dec 20. Clin Cancer Res. 2019. PMID: 30573690 Free PMC article. - Characterization of Postinfusion Phenotypic Differences in Fresh Versus Cryopreserved TCR Engineered Adoptive Cell Therapy Products.
Nowicki TS, Escuin-Ordinas H, Avramis E, Chmielowski B, Chodon T, Berent-Maoz B, Wang X, Kaplan-Lefko P, Yang L, Baltimore D, Economou JS, Ribas A, Comin-Anduix B. Nowicki TS, et al. J Immunother. 2018 Jun;41(5):248-259. doi: 10.1097/CJI.0000000000000216. J Immunother. 2018. PMID: 29470191 Free PMC article. - Potential use of T cell receptor genes to modify hematopoietic stem cells for the gene therapy of cancer.
Clay TM, Custer MC, Spiess PJ, Nishimura MI. Clay TM, et al. Pathol Oncol Res. 1999;5(1):3-15. doi: 10.1053/paor.1999.0003. Pathol Oncol Res. 1999. PMID: 10079371 Review. - Molecular Analysis of Elements of Melanoma Insensitivity to TCR-Engineered Adoptive Cell Therapy.
Jazirehi AR. Jazirehi AR. Int J Mol Sci. 2021 Oct 29;22(21):11726. doi: 10.3390/ijms222111726. Int J Mol Sci. 2021. PMID: 34769156 Free PMC article. Review.
Cited by
- Epigenetic Suppression of Transgenic T-cell Receptor Expression via Gamma-Retroviral Vector Methylation in Adoptive Cell Transfer Therapy.
Nowicki TS, Farrell C, Morselli M, Rubbi L, Campbell KM, Macabali MH, Berent-Maoz B, Comin-Anduix B, Pellegrini M, Ribas A. Nowicki TS, et al. Cancer Discov. 2020 Nov;10(11):1645-1653. doi: 10.1158/2159-8290.CD-20-0300. Epub 2020 Jul 22. Cancer Discov. 2020. PMID: 32699033 Free PMC article. - Reprogramming immune cells activity by furin-like enzymes as emerging strategy for enhanced immunotherapy in cancer.
François A, Descarpentrie J, Badiola I, Siegfried G, Evrard S, Pernot S, Khatib AM. François A, et al. Br J Cancer. 2023 Mar;128(7):1189-1195. doi: 10.1038/s41416-022-02073-1. Epub 2022 Dec 15. Br J Cancer. 2023. PMID: 36522477 Free PMC article. Review. - Understanding TCR affinity, antigen specificity, and cross-reactivity to improve TCR gene-modified T cells for cancer immunotherapy.
Spear TT, Evavold BD, Baker BM, Nishimura MI. Spear TT, et al. Cancer Immunol Immunother. 2019 Nov;68(11):1881-1889. doi: 10.1007/s00262-019-02401-0. Epub 2019 Oct 8. Cancer Immunol Immunother. 2019. PMID: 31595324 Free PMC article. Review. - Melanoma Metabolism: Cell Survival and Resistance to Therapy.
Luís R, Brito C, Pojo M. Luís R, et al. Adv Exp Med Biol. 2020;1219:203-223. doi: 10.1007/978-3-030-34025-4_11. Adv Exp Med Biol. 2020. PMID: 32130701 Review. - Chimeric Antigen Receptor (CAR) T Cell Therapy for Metastatic Melanoma: Challenges and Road Ahead.
Soltantoyeh T, Akbari B, Karimi A, Mahmoodi Chalbatani G, Ghahri-Saremi N, Hadjati J, Hamblin MR, Mirzaei HR. Soltantoyeh T, et al. Cells. 2021 Jun 9;10(6):1450. doi: 10.3390/cells10061450. Cells. 2021. PMID: 34207884 Free PMC article. Review.
References
- Dembic Z, Haas W, Zamoyska R, Parnes J, Steinmetz M, von Boehmer H. Transfection of the CD8 gene enhances T-cell recognition. Nature. 1987;326:510–511. - PubMed
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:917–924. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA129816/CA/NCI NIH HHS/United States
- M01 RR000865/RR/NCRR NIH HHS/United States
- U54 CA151819/CA/NCI NIH HHS/United States
- P50 CA086306/CA/NCI NIH HHS/United States
- R01 CA170689/CA/NCI NIH HHS/United States
- P01 CA132681/CA/NCI NIH HHS/United States
- CA-16042/CA/NCI NIH HHS/United States
- AI-28697/AI/NIAID NIH HHS/United States
- U54 CA119347/CA/NCI NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- P30 AI028697/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials