Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis - PubMed (original) (raw)
Review
Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis
Lei Liu et al. PLoS One. 2014.
Abstract
Background and aim: A large number of studies have tried to combine sorafenib with TACE for patients with unresectable hepatocellular carcinoma (HCC) and the results were controversial. We conducted this systematic review and meta-analysis to evaluate the safety and efficacy of combination therapy of sorafenib and TACE in the management of unresectable HCC.
Methods: MEDLINE, PsycINFO, Scopus, EMBASE, and the Cochrane Library were searched from January 1990 to October 2013 and these databases were searched for appropriate studies combining TACE and sorafenib in treatment of HCC. Two authors independently reviewed the databases and extracted the data and disagreements were resolved by discussion. Effective value and safety were analyzed. Effective value included disease control rate (DCR), time to progression (TTP) and overall survival (OS).
Results: 17 studies were included in the study. In the 10 noncomparative studies, DCR ranged from 18.4 to 91.2%. Median TTP ranged from 7.1 to 9.0 months, and median OS ranged from 12 to 27 months. In the 7 comparative studies, the hazard ratio (HR) for TTP was found to be 0.76 (95% CI 0.66-0.89; P<0.001) with low heterogeneity among studies (P = 0.243; I(2) = 25.5%). However, the HR for OS was found to be 0.81 (95% CI 0.65-1.01; P = 0.061) with low heterogeneity among studies (P = 0.259; I(2) = 25.4%). The common toxicities included fatigue, diarrhea, nausea, hand foot skin reaction (HFSR), hematological events, hepatotoxicity, alopecia, hepatotoxicity, hypertension and rash/desquamation. AEs are generally manageable with dose reductions.
Conclusions: Combination therapy may bring benefits for unresectable HCC patients in terms of TTP but not OS. Further well-designed randomized controlled studies are needed to confirm the efficacy of combination therapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Flow diagram of the study selection procedure.
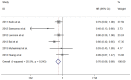
Figure 2. Forest plot showing the associations of the time to progression (TTP) between TACE alone group and sorafenib combined with TACE group for patients with unresctable HCC.
The result of meta-analysis for TTP between TACE alone group and sorafenib combined with TACE group for patients with unresctable HCC. Studies are arranged by publication year. Forrest plot displayed as hazard ratio and 95% confidence intervals. (HR, hazard ratio; CI, confidence interval).
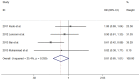
Figure 3. Forest plot showing the associations of the overall survival (OS) between TACE alone group and sorafenib combined with TACE group for patients with unresctable HCC.
The result of meta-analysis for OS between TACE alone group and sorafenib combined with TACE group for patients with unresctable HCC.Studies are arranged by publication year. Forrest plot displayed as hazard ratio and 95% confidence intervals. (HR, hazard ratio; CI, confidence interval).
Similar articles
- Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis.
Li L, Zhao W, Wang M, Hu J, Wang E, Zhao Y, Liu L. Li L, et al. BMC Gastroenterol. 2018 Sep 4;18(1):138. doi: 10.1186/s12876-018-0849-0. BMC Gastroenterol. 2018. PMID: 30180810 Free PMC article. Review. - Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis.
Yang M, Yuan JQ, Bai M, Han GH. Yang M, et al. Mol Biol Rep. 2014 Oct;41(10):6575-82. doi: 10.1007/s11033-014-3541-7. Epub 2014 Aug 5. Mol Biol Rep. 2014. PMID: 25091939 Review. - Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study.
Bai W, Wang YJ, Zhao Y, Qi XS, Yin ZX, He CY, Li RJ, Wu KC, Xia JL, Fan DM, Han GH. Bai W, et al. J Dig Dis. 2013 Apr;14(4):181-90. doi: 10.1111/1751-2980.12038. J Dig Dis. 2013. PMID: 23324079 Clinical Trial. - Combination trans arterial chemoembolization (TACE) plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review of the literature.
Abdel-Rahman O, Elsayed ZA. Abdel-Rahman O, et al. Dig Dis Sci. 2013 Dec;58(12):3389-96. doi: 10.1007/s10620-013-2872-x. Epub 2013 Sep 18. Dig Dis Sci. 2013. PMID: 24046163 Review. - Transarterial chemoembolization (TACE) plus sorafenib versus TACE for intermediate or advanced stage hepatocellular carcinoma: a meta-analysis.
Zhang L, Hu P, Chen X, Bie P. Zhang L, et al. PLoS One. 2014 Jun 19;9(6):e100305. doi: 10.1371/journal.pone.0100305. eCollection 2014. PLoS One. 2014. PMID: 24945380 Free PMC article.
Cited by
- Development of three-dimensional canine hepatic tumor model based on computed tomographic angiography for simulation of transarterial embolization.
Oh M, Ban J, Lee Y, Lee M, Kim S, Kim U, Park J, Han J, Chang J, Kim B, Yun H, Lee N, Chang D. Oh M, et al. Front Vet Sci. 2024 Jan 30;10:1280028. doi: 10.3389/fvets.2023.1280028. eCollection 2023. Front Vet Sci. 2024. PMID: 38352169 Free PMC article. - Diagnostics and Treatment of Hepatocellular Carcinoma in 2016: Standards and Developments.
Trojan J, Zangos S, Schnitzbauer AA. Trojan J, et al. Visc Med. 2016 Apr;32(2):116-20. doi: 10.1159/000445730. Epub 2016 Apr 12. Visc Med. 2016. PMID: 27413729 Free PMC article. Review. - Transarterial Chemoembolization within First 3 Months of Sorafenib Initiation Improves Overall Survival in Hepatocellular Carcinoma: A Retrospective, Multi-Institutional Study with Propensity Matching.
Kaplan DE, Mehta R, D'Addeo K, Gade TP, Taddei TH. Kaplan DE, et al. J Vasc Interv Radiol. 2018 Apr;29(4):540-549.e4. doi: 10.1016/j.jvir.2017.11.033. Epub 2018 Feb 22. J Vasc Interv Radiol. 2018. PMID: 29477619 Free PMC article. - Nanoquercetin and Extracellular Vesicles as Potential Anticancer Therapeutics in Hepatocellular Carcinoma.
Raghav A, Jeong GB. Raghav A, et al. Cells. 2024 Apr 5;13(7):638. doi: 10.3390/cells13070638. Cells. 2024. PMID: 38607076 Free PMC article. Review. - Hepatocellular carcinoma, novel therapies on the horizon.
El Dika I, Makki I, Abou-Alfa GK. El Dika I, et al. Chin Clin Oncol. 2021 Feb;10(1):12. doi: 10.21037/cco-20-113. Epub 2020 Jun 9. Chin Clin Oncol. 2021. PMID: 32527116 Free PMC article.
References
- Faloppi L, Scartozzi M, Maccaroni E, Di Pietro Paolo M, Berardi R, et al. (2011) Evolving strategies for the treatment of hepatocellular carcinoma: from clinical-guided to molecularly-tailored therapeutic options. Cancer treatment reviews 37: 169–177. - PubMed
- El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. The New England journal of medicine 340: 745–750. - PubMed
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA: a cancer journal for clinicians 55: 74–108. - PubMed
- Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology (Baltimore, Md) 42: 1208–1236. - PubMed
- Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, et al. (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. Journal of hepatology 35: 421–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
No current funding sources for this study.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous