Evaluation of the interaction between phosphohistidine analogues and phosphotyrosine binding domains - PubMed (original) (raw)
Evaluation of the interaction between phosphohistidine analogues and phosphotyrosine binding domains
Tom E McAllister et al. Chembiochem. 2014.
Abstract
We have investigated the interaction of peptides containing phosphohistidine analogues and their homologues with the prototypical phosphotyrosine binding SH2 domain from the eukaryotic cell signalling protein Grb2 by using a combination of isothermal titration calorimetry and a fluorescence anisotropy competition assay. These investigations demonstrated that the triazole class of phosphohistidine analogues are capable of binding too, suggesting that phosphohistidine could potentially be detected by this class of proteins in vivo.
Keywords: cell signaling; phosphohistidine; phosphotyrosine; protein modifications; synthetic analogues.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
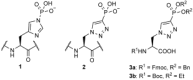
Scheme 1
Structures of τ-phosphohistidine (1) and the corresponding triazole-phosphonate mimic, 2, which can be incorporated into peptides by using precursors 3 a and 3 b.
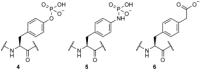
Scheme 2
Structures of phosphotyrosine 4 and analogues 5 and 6, which have previously been incorporated into recombinant proteins.
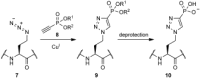
Scheme 3
Proposed route to generate a protein containing the putative phosphotyrosine analogue 10.
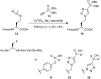
Scheme 4
Synthesis of Fmoc-/benzyl-protected homotriazole 14 from Fmoc-azidohomoalanine 12 and alkyne 13, as well as peptides 11, 15 and 16, which were used in binding experiments. For full experimental procedures see the Supporting information.
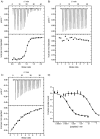
Figure 1
Isothermal titration calorimetry of peptides A) 11, B) 15 and C) 16 into the Grb2 SH2 domain reveals equilibrium binding constants of (385±41) n
m
for 11 and (719±28) μ
m
for 16. D) A competitive fluorescence polarisation assay indicates an IC50 of (442±80) n
m
for pTyr peptide 11 (▪) and (363±15) μ
m
for pTz peptide 16 (□). See the Supporting Information for control dilution experiments and full fitting of polarisation data.
Similar articles
- 3-Phosphohistidine cannot replace phosphotyrosine in high-affinity binding to phosphotyrosine binding or Src homology 2 domains.
Senderowicz L, Wang JX, Wang LY, Yoshizawa S, Kavanaugh WM, Turck CW. Senderowicz L, et al. Biochemistry. 1997 Aug 26;36(34):10538-44. doi: 10.1021/bi9707032. Biochemistry. 1997. PMID: 9265634 - Structural and energetic aspects of Grb2-SH2 domain-swapping.
Benfield AP, Whiddon BB, Clements JH, Martin SF. Benfield AP, et al. Arch Biochem Biophys. 2007 Jun 1;462(1):47-53. doi: 10.1016/j.abb.2007.03.010. Epub 2007 Apr 2. Arch Biochem Biophys. 2007. PMID: 17466257 Free PMC article. - Thermodynamic and structural analysis of phosphotyrosine polypeptide binding to Grb2-SH2.
McNemar C, Snow ME, Windsor WT, Prongay A, Mui P, Zhang R, Durkin J, Le HV, Weber PC. McNemar C, et al. Biochemistry. 1997 Aug 19;36(33):10006-14. doi: 10.1021/bi9704360. Biochemistry. 1997. PMID: 9254595 - Focus on phosphohistidine.
Attwood PV, Piggott MJ, Zu XL, Besant PG. Attwood PV, et al. Amino Acids. 2007 Jan;32(1):145-56. doi: 10.1007/s00726-006-0443-6. Epub 2006 Nov 15. Amino Acids. 2007. PMID: 17103118 Review. - Grb2 SH2 domain-binding peptide analogs as potential anticancer agents.
Lung FD, Tsai JY. Lung FD, et al. Biopolymers. 2003;71(2):132-40. doi: 10.1002/bip.10396. Biopolymers. 2003. PMID: 12767115 Review.
Cited by
- Phosphonopeptides containing free phosphonic groups: recent advances.
Kafarski P. Kafarski P. RSC Adv. 2020 Jul 9;10(43):25898-25910. doi: 10.1039/d0ra04655h. eCollection 2020 Jul 3. RSC Adv. 2020. PMID: 35518575 Free PMC article. Review. - pHisphorylation: the emergence of histidine phosphorylation as a reversible regulatory modification.
Fuhs SR, Hunter T. Fuhs SR, et al. Curr Opin Cell Biol. 2017 Apr;45:8-16. doi: 10.1016/j.ceb.2016.12.010. Epub 2017 Jan 25. Curr Opin Cell Biol. 2017. PMID: 28129587 Free PMC article. Review. - Advances in Fmoc solid-phase peptide synthesis.
Behrendt R, White P, Offer J. Behrendt R, et al. J Pept Sci. 2016 Jan;22(1):4-27. doi: 10.1002/psc.2836. J Pept Sci. 2016. PMID: 26785684 Free PMC article. Review. - Advances in development of new tools for the study of phosphohistidine.
Makwana MV, Muimo R, Jackson RF. Makwana MV, et al. Lab Invest. 2018 Mar;98(3):291-303. doi: 10.1038/labinvest.2017.126. Epub 2017 Dec 4. Lab Invest. 2018. PMID: 29200202 Review. - Strong anion exchange-mediated phosphoproteomics reveals extensive human non-canonical phosphorylation.
Hardman G, Perkins S, Brownridge PJ, Clarke CJ, Byrne DP, Campbell AE, Kalyuzhnyy A, Myall A, Eyers PA, Jones AR, Eyers CE. Hardman G, et al. EMBO J. 2019 Oct 4;38(21):e100847. doi: 10.15252/embj.2018100847. Epub 2019 Aug 21. EMBO J. 2019. PMID: 31433507 Free PMC article.
References
- Cohen P. Nature. 1982;296:613–620. - PubMed
- Pawson T, Scott JG. Science. 1997;278:2075–2080. - PubMed
- Schlessinger J, Lemmon MA. Sci. STKE. 2003;2003 re12. - PubMed
- Yaffe MB, Elia AE. Curr. Opin. Cell Biol. 2001;13:131–138. - PubMed
- Treharne KJ, Crawford RM, Mehta A. Exp. Physiol. 2006;91:131–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous