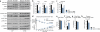Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression - PubMed (original) (raw)
. 2014 Jun 26;510(7506):547-51.
doi: 10.1038/nature13267. Epub 2014 May 25.
John E Dominy 2, Yoon Jong Choi 3, Michael Jurczak 4, Nicola Tolliday 5, Joao Paulo Camporez 4, Helen Chim 2, Ji-Hong Lim 2, Hai-Bin Ruan 4, Xiaoyong Yang 4, Francisca Vazquez 2, Piotr Sicinski 3, Gerald I Shulman 4, Pere Puigserver 2
Affiliations
- PMID: 24870244
- PMCID: PMC4076706
- DOI: 10.1038/nature13267
Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression
Yoonjin Lee et al. Nature. 2014.
Abstract
Insulin constitutes a principal evolutionarily conserved hormonal axis for maintaining glucose homeostasis; dysregulation of this axis causes diabetes. PGC-1α (peroxisome-proliferator-activated receptor-γ coactivator-1α) links insulin signalling to the expression of glucose and lipid metabolic genes. The histone acetyltransferase GCN5 (general control non-repressed protein 5) acetylates PGC-1α and suppresses its transcriptional activity, whereas sirtuin 1 deacetylates and activates PGC-1α. Although insulin is a mitogenic signal in proliferative cells, whether components of the cell cycle machinery contribute to its metabolic action is poorly understood. Here we report that in mice insulin activates cyclin D1-cyclin-dependent kinase 4 (Cdk4), which, in turn, increases GCN5 acetyltransferase activity and suppresses hepatic glucose production independently of cell cycle progression. Through a cell-based high-throughput chemical screen, we identify a Cdk4 inhibitor that potently decreases PGC-1α acetylation. Insulin/GSK-3β (glycogen synthase kinase 3-beta) signalling induces cyclin D1 protein stability by sequestering cyclin D1 in the nucleus. In parallel, dietary amino acids increase hepatic cyclin D1 messenger RNA transcripts. Activated cyclin D1-Cdk4 kinase phosphorylates and activates GCN5, which then acetylates and inhibits PGC-1α activity on gluconeogenic genes. Loss of hepatic cyclin D1 results in increased gluconeogenesis and hyperglycaemia. In diabetic models, cyclin D1-Cdk4 is chronically elevated and refractory to fasting/feeding transitions; nevertheless further activation of this kinase normalizes glycaemia. Our findings show that insulin uses components of the cell cycle machinery in post-mitotic cells to control glucose homeostasis independently of cell division.
Figures
Figure 1
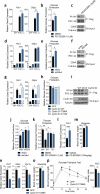
Cyclin D1-CDK4 modulates PGC-1α acetylation through GCN5. a) Scatter plot of chemicals plotted with first test z scores on the X-axis and repeated test scores on the Y-axis. b) Fascaplysin reduces PGC-1α acetylation and Rb phoshorylation. c) Fascaplysin and PD 0332991 treatments decrease PGC-1α acetylation. d) CDK4 knockdown causes PGC-1α deacetylation. e) GCN5 knockdown blunts fascaplysin-mediated PGC-1α deacetylation. f) GCN5 acetyltransferase activity is reduced upon fascaplysin treatment (n=2, mean±S.E.M). g) Endogenous GCN5 and CDK4 interact. h) Cyclin D1-CDK4 kinase phosphorylates GCN5 in vitro. i) GCN5 T272A/S372A (AA) phosphorylation by cyclin D1-CDK4 kinase is diminished compared to GCN5 wild-type (WT). j) GCN5 T272A/S372A displays decreased acetyltransferase capacity. k) GCN5 T272A/S372A has decreased acetyltransferase activity and insensitivity to fascaplysin treatment. Kinetic constants were calculated by Michaelis-Menten equation (n=4, AU/min=arbitrary unit/min, mean±S.E.M). U-2OS cells were used for these experiments.
Figure 2
Cyclin D1-CDK4 regulates gluconeogenesis in primary hepatocytes and whole animals. a-b) PD 0332991 increases gluconeogenic gene expression and glucose production (a: one-way ANOVA with Tukey post test, n=3, b: two-tailed unpaired t-test, n=8). c) PGC-1α acetylation is decreased by PD 0332991 treatment. d-e) CDK4 knockdown increases gluconeogenic gene expression and glucose production (d: oneway ANOVA with Tukey post test, n=6, e: two-tailed unpaired t-test, n=6). f) PGC-1α acetylation is decreased upon CDK4 deletion. g-h) Cyclin D1 wild-type decreases gluconeogenic gene expression, and cyclin D1 wild-type and cyclin D1 T286A, but not cyclin D1 K112E, repress glucose production (g: one-way ANOVA with Tukey post test, n=3/GFP, PGC-1α, n=6/Cyclin D1 WT and KE, h: one-way ANOVA with Dunnett post test, n=8). i) Overexpression of cyclin D1 wild-type (WT) and T286A (TA), but not cyclin D1 K112E (KE), induces PGC-1α acetylation. j) GCN5 knockdown blunts the increase of glucose production by CDK4 knockdown (two-tailed unpaired t-test, n=8). k) PD 0332991 increases glucose production with GCN5 wild-type (WT), but not with GCN5 T272A/S372A (AA) (one-way ANOVA with Newman-Keuls post test, n=8). l-m) PD 0332991 administration increases Pck1 gene expression and glycemia in mice (two-tailed unpaired t-test, n=18/vehicle, n=17/PD 0332991). n-o) Gluconeogenic gene expression and glycemia are reduced by cyclin D1 T286A overexpression in liver (two-tailed unpaired t-test, n=10/GFP, n=9/D1 T286A). p) Cyclin D1 T286A overexpression in liver decreases hepatic glucose production capacity assessed by pyruvate tolerance test (two-tailed unpaired t-test, n=20/GFP, n=24/D1 T286A AUC=area under curve). Statistical significance is represented by asterisk corresponding to *P<0.05, **P<0.01, ***P<0.001. Data are shown as mean±S.E.M.
Figure 3
Cyclin D1-CDK4 is regulated by insulin/GSK3β and hepatic cyclin D1 deletion causes increased gluconeogenesis and glycemia upon refeeding. a) Cyclin D1 gene expression is increased during refeeding (one-way ANOVA with Tukey post test, n=3/fast and10hr refed, n=4/4hr refed). b) Cyclin D1 protein and Rb phosphorylation are induced upon refeeding (N=nuclear and C=cytoplasmic liver extracts). c) Phosphorylation of GCN5 is increased upon refeeding. GCN5 was immunoprecipitated using anti-phosphoS*P (pS*P) antibody from livers infected with GFP or GCN5 adenovirus. d) Hepatocytes ploidy does not change upon fasting and refeeding (n=5). e) Ki-67 staining in liver shows no differences upon fasting and refeeding. Small intestine was used as a positive control. f) Nuclear cyclin D1 protein level is increased upon insulin and GSK3β inhibitors treatment in primary hepatocytes. All cells were infected with cyclin D1 wild-type adenovirus. g) Insulin and GSK3β inhibitors suppress gluconeogenic gene expression. All cells were infected with PGC-1α adenovirus (oneway ANOVA with Tukey post test, n=6). h) Phosphorylation of GCN5 is induced by insulin and blunted by PD 0332991 treatment. GCN5 was overexpressed whereas GFP was used as a negative control. i) Cyclin D1 is transcriptionally induced by dietary intake of amino acids. Mice were fasted overnight or refed 4hr with chow diet, empty calorie, glucose or glucose and amino acids diet (one-way ANOVA with Tukey post test, n=5). j-k) Liver-specific cyclin D1 KO (D1 LKO) mice exhibit no differences on gluconeogenic gene expression and glycemia during fasting. l-m) D1 LKO mice display increased gluconeogenic gene expression and glycemia upon 4hr refeeding (one-way ANOVA with Tukey post test, combined 4 cohorts of n=3/fasting, n=5/refeeding). n) D1 LKO mice show mild glucose intolerance (two-way ANOVA, multiple comparison, n=11/WT1, n=7/WT2, n=10/D1 LKO). o) D1 LKO mice exhibit mild insulin intolerance (two-way ANOVA, significant interaction, multiple comparison, n=10/WT1, n=7/WT2, n=9/D1 LKO). Statistical significance is represented by asterisk corresponding to *P<0.05, **P<0.01, ***P<0.001. Data are shown as mean±S.E.M.
Figure 4
In diabetic hyperinsulinemic mice, cyclin D1-CDK4 is dysregulated and hyperactivation of cyclin D1-CDK4 attenuates the diabetic phenotype. a) Cyclin D1 is chronically elevated in livers of db/db mice (N=nuclear and C=cytoplasmic liver extracts). b-c) Cyclin D1 T286A, but not cyclin D1 K112E, overexpression in liver represses gluconeogenic genes and glycemia in db/db mice (one-way ANOVA, Tukey post test, n=6/GFP, D1 K112E, n=5/D1 T286A). d-h) In db/db mice, cyclin D1 T286A overexpression in liver suppresses hepatic glucose production tested by hyperinsulinemic-euglycemic clamp. d) Plasma glycemia and glucose infusion rate (twoway ANOVA, significant interaction, n=7). e) Body weights. f) Clamped glucose infusion rate. g) Whole-body glucose uptake. h) Hepatic glucose output (average of last 40min values for f-g, two-tailed unpaired t-test for d-h, n=7). Statistical significance is represented by asterisk corresponding to *P<0.05, **P<0.01, ***P<0.001. Data are shown as mean±S.E.M.
Extended Data Figure 1
A cell-based high throughput screen reveals compounds regulating PGC-1α acetylation. a) Scheme of high throughput chemical assay. b) Compounds with significant z scores either >3.0 or <-3.0 are listed. Inhibitors indicate the compounds that increased PGC-1α acetylation while activators indicate the ones that decreased PGC-1α acetylation.
Extended Data Figure 2
Cyclin D1-CDK4 modulates PGC-1α acetylation through GCN5. a) Fascaplysin decreases PGC-1α acetylation in dose-dependent manner. Dose-dependent response of PGC-1α acetylation treated with fascaplysin concentrations ranging from 31.25nM to 8μM. IC50 value was calculated using three independent measurements from the assay described in Extended Data Fig. 1 a. b) Chemical structures of fascaplysin and PD 0332991. c) CDK4 is knockdown by various CDK4 shRNAs used in Fig. 1d. d) Fascaplysin decreases PGC-1α acetylation upon EX527 or trichostatin A treatments. Cells were treated for 8hr in case of 1μM fascaplysin and 4hr in case of 1μM EX527 or trichostatin A prior to harvest. DMSO (-) was used as a control treatment. e) Fascpalysin has blunted effect on PCAF-mediated PGC-1α acetylation. f) Ectopically expressed CDK4 and GCN5 interact. As a comparison, PGC-1α, Sirt1 and Sirt6 were used while GFP was overexpressed as a negative control. g) Phosphorylation of GCN5 by cyclin D1-CDK4 complex is reduced by fascaplysin. DMSO or 1μM fascaplysin was added to the kinase reaction. h) In vitro phosphorylation of GST-GCN5 recombinant proteins (1-224aa, 1-386aa, 1-553aa, 1-837aa) by cyclin D1-CDK4 and the protein level of those fragments. i) GCN5 wild-type (WT), treated with fascaplysin and GCN5 T272A/S372A (AA) mutant immunoprecipitated by anti-phospho-S*P (pS*P) antibody. j) Acetylation of PGC-1α closely follows the amount of PAF65β bound to GCN5. Nuclear extracts of U-2OS overexpressing various amounts of GCN5 were used for western-blot analysis to detect GCN5 and PAF65β. Empty vector was transfected as a negative control. k) Interaction between GCN5 T272A/S372A (AA) and PAF65β is reduced compared to GCN5 wild-type (WT). U-2OS cells were used for western-blot analysis experiments.
Extended Data Figure 3
Cyclin D1-CDK4 regulates gluconeogenesis in primary hepatocytes and in whole animals. a) Western-blot analysis of endogenous, forskolin-induced (Fsk) or adenovirally overexpressed (O/E) PGC-1α. Nuclear extracts of primary hepatocytes were used to immunoprecipitate PGC-1α. Cells were infected with GFP or PGC-1α 48hr prior to harvest. 10μM forskolin was added for 2hr before harvest. b) PD 0332991 increases forskolin-induced gluconeogenic gene expression. Primary hepatocytes were treated with 10μM forskolin for 1.5hr following 3hr of starvation medium incubation while 1μM PD 0332991 was added overnight (one-way ANOVA with Tukey post test, n=3). c) CDK4 knockdown increases forskolin-induced gluconeogenic gene expression (one-way ANOVA with Tukey post test, n=3). d) Cyclin D1 wild-type, but not cyclin D1 K112E mutant, suppresses forskolin-induced gluconeogenic gene expression (one-way ANOVA with Tukey post test, n=3). e) Phosphorylation of GCN5, FoxO1 N-terminus, FoxO1 C-terminus, FoxO3A and PGC-1α SR domain by cyclin D1-CDK4. f) PGC-1α knockdown blocks the increase of forskolin-induced gluconeogenic genes by fascaplysin in HepG2 cells. PGC-1α knockdown or a negative control HepG2 cells were treated with 30μM forskolin and 1μM fascaplysin overnight (one-way ANOVA with Tukey post test, n=3). g) GCN5 knockdown blunts the increase of gluconeogenic gene expression caused by CDK4 knockdown. qRT-PCR analysis of Pck1 and Gcn5 and western-blot of CDK4 and GCN5 knockdown are shown. All cells were infected with PGC-1α adenoviruses (one-way ANOVA with Tukey post test, n=15). h) PD 0332991 increases gluconeogenic genes when combined with GCN5 wild-type (WT) overexpression, but not with GCN5 T272A/S372A (AA) mutant. GFP infected cells shown as a comparison to GCN5 overexpressing cells. All cells were infected with PGC-1α adenoviruses (two-tailed unpaired t-test, n=6). I-j) Insulin levels measured from serum and western-blot analysis of Rb and AKT using nuclear (N) and cytoplasmic (C) liver extracts from mice treated with vehicle or 150mg/kg PD 0332991, shown in Fig. 2lm (i: two-tailed unpaired t-test, n=18/GFP, n=17/PD 0332991). k) Levels of cyclin D1 and Rb phosphorylation in GFP or Cyclin D1 T286A tail-vein injected mice, shown in Fig. 2n-o. Statistical significance is represented by asterisk corresponding to *P<0.05, **P<0.01, ***P<0.001. Data are shown as mean±S.E.M.
Extended Data Figure 4
PD 0332991 administration or cyclin D1 T286A adenoviral overexpression does not cause toxicity compared to its respective control treatment. a) Basal physiological indexes of mice challenged with either vehicle or PD 0332991 administration (n=5). b) Basal physiological indexes of mice injected with either GFP or cyclin D1 T286A adenoviruses (n=5). (ALT=alanine transaminase, AST=aspartate transaminase, LDH=lactate dehydrogenase, mean±S.E.M)
Extended Data Figure 5
Cyclin D1-CDK4 is regulated by insulin/GSK3β and hepatic specific cyclin D1 deletion causes increased gluconeogenesis and glycemia upon refeeding. a) Cyclin D1 transcripts are increased upon refeeding. qRT-PCR analysis of Ccnd1, Ccnd2 and Ccne1 gene expression upon overnight fasting, 4hr and 10hr refeeding in BALB/c mice livers (one-way ANOVA with Tukey post test, n=3). b) Cyclin D1 protein is increased upon refeeding. Western-blot analysis of cyclin D1 protein levels and associated signaling pathway upon fasting and refeeding measured from nuclear (N) and cytoplasmic (C) liver extracts from BALB/c mice. c) Cyclin D1-CDK4 kinase activity is increased upon 4hr refeding. In vitro 32P incorporation into recombinant Rb by immunoprecipitated cyclin D1-CDK4 kinase from whole-cell extracts of overnight fast and 4hr refed livers. d) Western-blot analysis of cyclin D1 protein level and associated signaling pathway upon fasting and refeeding and qRT-PCR analysis of Ccnd1 and Pgc1α mRNA level in various tissues (L=liver, M=skeletal muscle, B=brown adipose tissue, W=epididymal white adipose tissue, and P=pancreas, two-tailed unpaired t-test, n=12/L, n=4/M, B, W, P). e) qRT-PCR analysis of PGC-1α target genes in liver and epididymal white adipose tissues (eWAT) upon vehicle or PD 0332991 treatment (two-tailed unpaired t-test, n=10). f) Ccnb1 and Pcna gene expressions in liver do not change upon fasting and refeeding (n=3/fast and 10hr refed, n=4/4hr refed). g) BrdU incorporation in liver does not change upon fasting and refeeding. Small intestine was used as a positive control. h) Ki-67 staining in liver does not change upon fasting and refeeding following vehicle or PD 0332991 administration. Small intestine used as a positive control. i) Ki-67 staining in liver does not change upon fasting and refeeding following GFP or cyclin D1 T286A tail-vein injection. Small intestine used as a positive control. j) Hepatic ploidy profiles of livers of GFP or cyclin D1 T286A adenovirus tail-vein injected mice do not show significant difference. Ploidy analysis of primary hepatocytes isolated from livers measured by propidium iodide staining and flow cytometry (n=6/fast and 4hr refed, n=4/10hr refed). k) Western-blot analysis of endogenous nuclear (N) and cytoplasmic (C) cyclin D1 protein level upon insulin or GSK3β inhibitors treatments in primary hepatocytes. l) PGC-1α acetylation is increased upon insulin or GSK3β inhibitors treatment in primary hepatocytes. m) No effect of insulin or GSK3β inhibitors on cyclin D1 mRNA level (n=3). n) Minimum essential medium (MEM) amino acids addition increases cyclin D1 mRNA in primary hepatocytes (one-way ANOVA with Tukey post test, n=3). o) Insulin does not change Ccnd1 mRNA in primary hepatocytes (oneway ANOVA with Tukey post test, n=3). p-q) Body, liver weights and Ccnd1 and Ccnd2 gene expression of wild-type and liver-specific cyclin D1 KO (D1 LKO) mice (combined 4 cohorts of n=3/fasting, n=5/refeeding). r) Western-blot analysis of cyclin D1 protein levels and associated signaling pathway by using nuclear and cytoplasmic liver extracts from wild-type and D1 LKO mice upon fasting (F) and 4hr refeeding (R). s) Endogenous acetylation of PGC-1α is decreased in livers of D1 LKO mice compared to wild-type mice. Western-blot analysis of acetylation of PGC-1α immunoprecipitated from liver nuclear extracts. All mice were sacrificed upon 4hr refeeding. t-u) PD 0332991 increases glycemia with similar tendency for gluconeogenic gene expression only in wild-type mice, but not in D1 LKO mice (two-tailed unpaired t-test, n=8, except n=6 for vehicle treated wild-type mice). v-w) Gluconeogenic gene expression and hepatic glucose production are increased in primary hepatocytes isolated from D1 LKO mice (v: one-way ANOVA Tukey post test, n=3, w: two-tailed unpaired test, n=6). Statistical significance is represented by asterisk corresponding to *P<0.05, **P<0.01, ***P<0.001. Data are shown as mean±S.E.M.
Extended Data Figure 6
In diabetic hyperinsulinemic mice, cyclin D1-CDK4 is dysregulated and hyperactivation of cyclin D1-CDK4 attenuates the diabetic phenotype. a) qRT-PCR analysis of gluconeogenic and Ccnd1 gene expression changes upon fasting and refeeding in Leprdb/+ (db/+) and Leprdb/db (db/db) mice livers (two-tailed unpaired t-test, n=3). b) Cyclin D1 protein is chronically elevated upon fasting and refeeding in livers of high fat diet fed mice compared to control diet fed mice. Nuclear (N) and cytoplasmic (C) liver extracts were used. c-d) Phosphorylation of GCN5 is elevated upon fasting in db/db or high fat diet fed (HFD) mice liver compared its respective control mice and it remains insensitive to fast-refed transitions. Western-blot analysis of GCN5 immnunoprecipiated by anti-phosphoS*P (pS*P) antibody using liver extracts from mice that were tail-vein injected with adenoviruses expressing GFP or GCN5 (F=16hr fast, R=4hr refed). e) Cyclin D1 and Rb phosphorylation levels in livers from db/db mice tail-vein injected with GFP, cyclin D1 T286A, or cyclin D1 K112E adenoviruses, shown in Fig. 4b-c. Nuclear and cytoplasmic liver extracts were used. f-g) Cyclin D1 T286A overexpression reduces gluconeogenic genes and glycemia in high fat diet fed mice (two-tailed unpaired t-test, n=6/GFP, n=7/D1 T286A). h) Cyclin D1 and Rb phosphorylation levels in livers of high fat diet fed mice that were tail-vein injected with adenoviruses expressing GFP or cyclin D1 T286A, shown in Extended Data Fig. 4f-g. i) Overall Model. Statistical significance is represented by asterisk corresponding to *P<0.05, **P<0.01, ***P<0.001. Data are shown as mean±S.E.M.
Similar articles
- Cyclin D1 represses gluconeogenesis via inhibition of the transcriptional coactivator PGC1α.
Bhalla K, Liu WJ, Thompson K, Anders L, Devarakonda S, Dewi R, Buckley S, Hwang BJ, Polster B, Dorsey SG, Sun Y, Sicinski P, Girnun GD. Bhalla K, et al. Diabetes. 2014 Oct;63(10):3266-78. doi: 10.2337/db13-1283. Epub 2014 Jun 19. Diabetes. 2014. PMID: 24947365 Free PMC article. - CITED2 links hormonal signaling to PGC-1α acetylation in the regulation of gluconeogenesis.
Sakai M, Matsumoto M, Tujimura T, Yongheng C, Noguchi T, Inagaki K, Inoue H, Hosooka T, Takazawa K, Kido Y, Yasuda K, Hiramatsu R, Matsuki Y, Kasuga M. Sakai M, et al. Nat Med. 2012 Mar 18;18(4):612-7. doi: 10.1038/nm.2691. Nat Med. 2012. PMID: 22426420 - The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis.
Dominy JE Jr, Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, Ruan HB, Feldman J, Pierce K, Mostoslavsky R, Denu JM, Clish CB, Yang X, Shulman GI, Gygi SP, Puigserver P. Dominy JE Jr, et al. Mol Cell. 2012 Dec 28;48(6):900-13. doi: 10.1016/j.molcel.2012.09.030. Epub 2012 Nov 8. Mol Cell. 2012. PMID: 23142079 Free PMC article. - GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy.
Takahashi-Yanaga F, Sasaguri T. Takahashi-Yanaga F, et al. Cell Signal. 2008 Apr;20(4):581-9. doi: 10.1016/j.cellsig.2007.10.018. Epub 2007 Oct 23. Cell Signal. 2008. PMID: 18023328 Review. - CAT in the HAT: catabolic inhibition by the histone acetyltransferase GCN5.
Liu Y, Montminy M. Liu Y, et al. Cell Metab. 2006 Jun;3(6):387-8. doi: 10.1016/j.cmet.2006.05.006. Cell Metab. 2006. PMID: 16753572 Review.
Cited by
- Nuclear metabolism and the regulation of the epigenome.
Boon R, Silveira GG, Mostoslavsky R. Boon R, et al. Nat Metab. 2020 Nov;2(11):1190-1203. doi: 10.1038/s42255-020-00285-4. Epub 2020 Oct 12. Nat Metab. 2020. PMID: 33046909 Review. - Overexpression of interleukin-18 protein reduces viability and induces apoptosis of tongue squamous cell carcinoma cells by activation of glycogen synthase kinase-3β signaling.
Liu W, Hu M, Wang Y, Sun B, Guo Y, Xu Z, Li J, Han B. Liu W, et al. Oncol Rep. 2015 Mar;33(3):1049-56. doi: 10.3892/or.2015.3724. Epub 2015 Jan 15. Oncol Rep. 2015. PMID: 25591548 Free PMC article. - Postmitotic G1 phase survivin drives mitogen-independent cell division of B lymphocytes.
Singh A, Spitzer MH, Joy JP, Kaileh M, Qiu X, Nolan GP, Sen R. Singh A, et al. Proc Natl Acad Sci U S A. 2022 May 3;119(18):e2115567119. doi: 10.1073/pnas.2115567119. Epub 2022 Apr 27. Proc Natl Acad Sci U S A. 2022. PMID: 35476510 Free PMC article. - DHA Suppresses Hepatic Lipid Accumulation via Cyclin D1 in Zebrafish.
Ding Q, Hao Q, Zhang Q, Yang Y, Olsen RE, Ringø E, Ran C, Zhang Z, Zhou Z. Ding Q, et al. Front Nutr. 2022 Jan 25;8:797510. doi: 10.3389/fnut.2021.797510. eCollection 2021. Front Nutr. 2022. PMID: 35145984 Free PMC article. - lncRNA LINC01296 regulates the proliferation, metastasis and cell cycle of osteosarcoma through cyclin D1.
Yu X, Pang L, Yang T, Liu P. Yu X, et al. Oncol Rep. 2018 Nov;40(5):2507-2514. doi: 10.3892/or.2018.6674. Epub 2018 Aug 30. Oncol Rep. 2018. PMID: 30226542 Free PMC article.
References
- Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. The Journal of biological chemistry. 1999;274:15982–15985. - PubMed
- Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 2001;292:1728–1731. doi:10.1126/science.292.5522.1728. - PubMed
- Dentin R, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi:10.1038/nature06128. - PubMed
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell metabolism. 2007;6:208–216. doi:10.1016/j.cmet.2007.08.006. - PubMed
- Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi:10.1038/nature01667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA083688/CA/NCI NIH HHS/United States
- R01 DK089098/DK/NIDDK NIH HHS/United States
- R03 MH092174/MH/NIMH NIH HHS/United States
- R03 DA032468/DA/NIDA NIH HHS/United States
- F32 DK083871/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- R24 DK080261/DK/NIDDK NIH HHS/United States
- R01 CA108420/CA/NCI NIH HHS/United States
- R01 DK069966/DK/NIDDK NIH HHS/United States
- R01069966/PHS HHS/United States
- R24DK080261-06/DK/NIDDK NIH HHS/United States
- DK059635/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous