The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis - PubMed (original) (raw)
Review
The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis
Piotr Wojdasiewicz et al. Mediators Inflamm. 2014.
Abstract
Osteoarthritis (OA) is the most common chronic disease of human joints. The basis of pathologic changes involves all the tissues forming the joint; already, at an early stage, it has the nature of inflammation with varying degrees of severity. An analysis of the complex relationships indicates that the processes taking place inside the joint are not merely a set that (seemingly) only includes catabolic effects. Apart from them, anti-inflammatory anabolic processes also occur continually. These phenomena are driven by various mediators, of which the key role is attributed to the interactions within the cytokine network. The most important group controlling the disease seems to be inflammatory cytokines, including IL-1 β , TNF α , IL-6, IL-15, IL-17, and IL-18. The second group with antagonistic effect is formed by cytokines known as anti-inflammatory cytokines such as IL-4, IL-10, and IL-13. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of OA with respect to inter- and intracellular signaling pathways is still under investigation. This paper summarizes the current state of knowledge. The cytokine network in OA is put in the context of cells involved in this degenerative joint disease. The possibilities for further implementation of new therapeutic strategies in OA are also pointed.
Figures
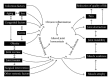
Figure 1
Schematic of a closed disease circle comprising the disease progression of osteoarthritis taking into account its causes and consequences.
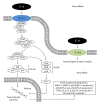
Figure 2
IL-1_β_ associated intracellular signaling pathways and downstream cellular targets and effects. IL-1R1: interleukin-1 receptor, type 1; IL-1R2: interleukin-1 receptor, type 2; MyD88: myeloid differentiation primary response gene (88); IRAK: interleukin-1 receptor-associated kinase; TRAF6: TNF receptor-associated factor 6; TAK1: also known as mitogen-activated protein kinase kinase kinase 7 (MAP3K7); TAB1: also known as mitogen-activated protein kinase kinase kinase 7 interacting protein 1 (MAP3K7IP1); TAB2: also known as mitogen-activated protein kinase kinase kinase 7 interacting protein 2 (MAP3K7IP2); p50, p65: subunits of proteins forming NF-_κ_B; I_κ_B: (inhibitor of _κ_B) an endogenous complex of proteins inhibiting the activation of NF-_κ_B; IKK1,2/NEMO: NF-_κ_B inhibitor kinase 1,2 (I_κ_B kinase 1,2)/NF-_κ_B kinase inhibitor (NF-_κ_B essential modulator); ERK: extracellular-signal-regulated kinase; JNK: c-Jun N-terminal kinase; p38: p38 mitogen-activated protein kinases; MAPK: mitogen-activated protein kinases; AP-1: activator protein 1.
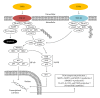
Figure 3
TNF_α_ associated intracellular signaling pathways and downstream cellular targets and effects. TNF-R1: tumor necrosis factor receptor superfamily member 1; TNF-R2: tumor necrosis factor receptor superfamily member 2; TRADD: tumor necrosis factor receptor type 1 associated death domain protein; FADD: Fas-associated protein with death domain; TRAF2: TNF receptor-associated factor 6; c-IAP1: also known as Baculoviral IAP repeat-containing protein 2 (BIRC2); c-IAP2: also known as Baculoviral IAP repeat-containing protein 3 (BIRC3); RIP1: receptor-interacting protein kinase 1; Ub: ubiquitin; TRAF3: TNF receptor-associated factor 3; TAK1: also known as mitogen-activated protein kinase kinase kinase 7 (MAP3K7); TAB1: also known as mitogen-activated protein kinase kinase kinase 7 interacting protein 1 (MAP3K7IP1); TAB2: also known as mitogen-activated protein kinase kinase kinase 7 interacting protein 2 (MAP3K7IP2); p50, p65: subunits of proteins forming NF-_κ_B; I_κ_B: (inhibitor of _κ_B) an endogenous complex of proteins inhibiting the activation of NF-_κ_B; IKK1,2/NEMO: NF-_κ_B inhibitor kinase 1,2 (I_κ_B kinase 1,2)/NF-_κ_B kinase inhibitor (NF-_κ_B essential modulator); ERK: extracellular-signal-regulated kinase; JNK: c-Jun N-terminal kinase; p38: p38 mitogen-activated protein kinases; MAPK: mitogen-activated protein kinases; AP-1: activator protein 1.
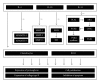
Figure 4
Schematic of the anti-inflammatory and chondroprotective effect of IL-4, IL-10, and IL-13 on articular cartilage during the course of OA.
Similar articles
- Anticytokine therapy for osteoarthritis: evidence to date.
Malemud CJ. Malemud CJ. Drugs Aging. 2010 Feb 1;27(2):95-115. doi: 10.2165/11319950-000000000-00000. Drugs Aging. 2010. PMID: 20104937 - Cytokines in the pathogenesis of hemophilic arthropathy.
Wojdasiewicz P, Poniatowski ŁA, Nauman P, Mandat T, Paradowska-Gorycka A, Romanowska-Próchnicka K, Szukiewicz D, Kotela A, Kubaszewski Ł, Kotela I, Kurkowska-Jastrzębska I, Gasik R. Wojdasiewicz P, et al. Cytokine Growth Factor Rev. 2018 Feb;39:71-91. doi: 10.1016/j.cytogfr.2017.11.003. Epub 2017 Nov 13. Cytokine Growth Factor Rev. 2018. PMID: 29153709 Review. - Osteoarthritis: can anti-cytokine therapy play a role in treatment?
Calich AL, Domiciano DS, Fuller R. Calich AL, et al. Clin Rheumatol. 2010 May;29(5):451-5. doi: 10.1007/s10067-009-1352-3. Epub 2010 Jan 27. Clin Rheumatol. 2010. PMID: 20108016 Review.
Cited by
- The activation of IL-17 signaling pathway promotes pyroptosis in pneumonia-induced sepsis.
Li LL, Dai B, Sun YH, Zhang TT. Li LL, et al. Ann Transl Med. 2020 Jun;8(11):674. doi: 10.21037/atm-19-1739. Ann Transl Med. 2020. PMID: 32617294 Free PMC article. - Protective Effects of Phellinus linteus Mycelium on the Development of Osteoarthritis after Monosodium Iodoacetate Injection.
Shin MR, Lee JA, Kim MJ, Park HJ, Park BW, Seo SB, Roh SS. Shin MR, et al. Evid Based Complement Alternat Med. 2020 Aug 15;2020:7240858. doi: 10.1155/2020/7240858. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32908566 Free PMC article. - SOCS1 suppresses IL-1β-induced C/EBPβ expression via transcriptional regulation in human chondrocytes.
Ha YJ, Choi YS, Kang EH, Shin K, Kim TK, Song YW, Lee YJ. Ha YJ, et al. Exp Mol Med. 2016 Jun 24;48(6):e241. doi: 10.1038/emm.2016.47. Exp Mol Med. 2016. PMID: 27339399 Free PMC article. - Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols.
Ansari MY, Ahmad N, Haqqi TM. Ansari MY, et al. Biomed Pharmacother. 2020 Sep;129:110452. doi: 10.1016/j.biopha.2020.110452. Epub 2020 Jul 3. Biomed Pharmacother. 2020. PMID: 32768946 Free PMC article. Review. - Association of Serum Biochemical Biomarker Profiles of Joint Tissue Inflammation and Cartilage Metabolism With Posttraumatic Osteoarthritis-Related Symptoms at 12 Months After ACLR.
Lisee C, Obudzinski S, Pietrosimone BG, Alexander Creighton R, Kamath G, Longobardi L, Loeser R, Schwartz TA, Spang JT. Lisee C, et al. Am J Sports Med. 2024 Aug;52(10):2503-2511. doi: 10.1177/03635465241262797. Epub 2024 Aug 11. Am J Sports Med. 2024. PMID: 39129267 Free PMC article.
References
- Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. The Lancet. 2011;377(9783):2115–2126. - PubMed
- Madry H, Cucchiarini M. Advances and challenges in gene-based approaches for osteoarthritis. The Journal of Gene Medicine. 2013;15(10):343–355. - PubMed
- de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484–1499. - PubMed
- Vangsness CT, Jr., Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—a pilot study. Bulletin of the NYU Hospital for Joint Diseases. 2011;69(2):122–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous