Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry - PubMed (original) (raw)
. 2014 Jun 16;25(6):831-45.
doi: 10.1016/j.ccr.2014.04.016. Epub 2014 May 29.
Maria Sol Degese 2, Ramiro Iglesias-Bartolome 2, Jose P Vaque 2, Alfredo A Molinolo 2, Murilo Rodrigues 3, M Raza Zaidi 4, Bruce R Ksander 5, Glenn Merlino 6, Akrit Sodhi 3, Qianming Chen 7, J Silvio Gutkind 8
Affiliations
- PMID: 24882515
- PMCID: PMC4074519
- DOI: 10.1016/j.ccr.2014.04.016
Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry
Xiaodong Feng et al. Cancer Cell. 2014.
Abstract
Mutually exclusive activating mutations in the GNAQ and GNA11 oncogenes, encoding heterotrimeric Gαq family members, have been identified in ∼ 83% and ∼ 6% of uveal and skin melanomas, respectively. However, the molecular events underlying these GNAQ-driven malignancies are not yet defined, thus limiting the ability to develop cancer-targeted therapies. Here, we focused on the transcriptional coactivator YAP, a critical component of the Hippo signaling pathway that controls organ size. We found that Gαq stimulates YAP through a Trio-Rho/Rac signaling circuitry promoting actin polymerization, independently of phospholipase Cβ and the canonical Hippo pathway. Furthermore, we show that Gαq promotes the YAP-dependent growth of uveal melanoma cells, thereby identifying YAP as a suitable therapeutic target in uveal melanoma, a GNAQ/GNA11-initiated human malignancy.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
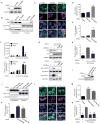
Figure 1. Activating mutations in Gαq (GαqQL) induces YAP nuclear translocation and YAP-Dependent Transcription activation through Trio and Trio dependent Rho-GTPases
(A) Western blots show HA-Gαq and HA-GαqQL expression in HEK293 cells transfected with HA-Gαq or HA-GαqQL expression vectors (DNAs), using endogenous GAPDH as a loading control. (B) Western blot shows YAP expression levels in the nuclear fraction; enrichment for Lamin A/C and α-Tubulin served as nuclear and cytoplasmic markers respectively. (C and D) Immunofluorescence shows that transfected GαqQL induces YAP nuclear translocation, but not Gαq or mCherry. (C) Endogenous YAP (green) was detected by immunofluorescence along with Hoechst for nuclear DNA (blue) and HA staining (violet) or mCherry (violet, as control). (D) Nuclear YAP in HA-positive and mCherry-positive cells was quantified with Image-J and represented as arbitrary units in the indicated cell populations (mean ± SEM, n = 50–100 cells). (E) HEK293 cells were cotransfected with HA-Gαq or HA-GαqQL and Gal4-TEAD4, 5×UAS-Luc and Renilla-Luc DNAs followed by luciferase assay (mean ± SEM, n = 3). (F) HEK293 cells were transfected with HA-Gαq or HA-GαqQL, followed by posphoinositide (PI) turnover assays (mean ± SEM, n = 6) (upper panel) or cotransfected with Gal4-TEAD4, 5×UAS-Luc and Renilla-Luc DNAs, followed by PLCi treatment (1 hr) and luciferase assay (mean ± SEM, n = 3) (lower panel). (G) Transfected HA-GαqQL or vector into shRNA-control, shRNA-Trio#1 and shRNA–Trio#2 HEK293 cells, followed by the indicated Western blot analysis (upper panel) or by RhoA and Rac1 small GTPases activation assays (lower panels). (H) HEK293 cells were cotransfected with siRNA Trio or control and HA-GαqQL or vector and Gal4-TEAD4, 5×UAS-Luc and Renilla-Luc DNAs, followed by luciferase assay (mean ± SEM, n = 6). (I) Western blot show AU5-RhoAQL and AU5-Rac1QL expression in HEK293 cells transfected with the corresponding expression plasmids. (J) Western blots show that both RhoAQL and Rac1QL can induce YAP accumulation in the nuclear fraction, using enrichment in Lamin A/C and α-Tubulin as nuclear and cytoplasmic markers respectively. (K) HEK293 cells were cotransfected with AU5-RhoAQL or AU5-Rac1QL and Gal4-TEAD4, 5×UAS-Luc and Renilla-Luc DNAs, followed by luciferase assays (mean ± SEM, n = 6). (L and M) Immunofluorescence assay and nuclear YAP quantification, using the procedure described in 1C in the indicated transfected cells (mean ± SEM, n = 50–100 cells). (N) HEK293 cells were cotransfected with siRNAs RhoA, Rac1 or control and HA-GαqQL or vector and Gal4-TEAD4, 5×UAS-Luc and Renilla-Luc DNAs, followed by luciferase assay, as above (mean ± SEM, n = 6). See also Figure S1.
Figure 2. Conditional expression of the GαqQL promotes melanoma or skin carcinoma formation and YAP activation in vivo
(A) Dct-rtTA mice were bred with tet-H2BGFP transgenic mice to produce inducible Dct/H2BGFP double transgenic mice, which express GFP exclusively in melanocytes, when fed with doxycycline food (dox). (B) Dct/H2BGFP mice show tight regulation GFP expression in skin melanocytes (as showed in Zaidi et al., 2011) (C) _Dct-rtTA/p16p19_KO mice were bred with _tet-HA-GαqQL/p16p19_KO mice to produce inducible _Dct/HA-GαqQL/p16p19_KO mice, which expressed HA-GαqQL exclusively in melanocytes, when fed with doxycycline food (dox). (D) Percentage of mice developing cutaneous lesions of melanocytic origin after feeding with doxycycline food. (E) Example of Dct/HA-GαqQL/p16p19KO mice developing lesions in the skin. (F) Histology shows _Dct/HA-GαqQL/p16p19_KO mouse with cutaneous melanoma (upper right panel). Immunofluorescence assay of frozen tissues shows that HA-GαqQL (green) positive cells display YAP (violet) nuclear translocation using Hoechst for DNA staining (blue) (right lower panel). Normal skin from Dct/H2BGFP mouse stained with GFP (green) instead of HA as control (left lower panel) shows cytoplasmic YAP. (G) Quantification of percent nuclear YAP positive cells in GFP or HA positive cells (GFP+ and HA+, respectively). (H) K5-rtTA mice were bred with tet-O-HA-GαqQL mice to produce inducible K5/HA-GαqQL mice, which express HA-GαqQL exclusively in basal epithelial cells (Vitale-Cross et al., 2004), when fed with doxycycline food (dox). (I) K5/HA-GαqQL mice developed rapid hair loss within days (left), and exhibited multiple tumor lesions on the skin (right). (J) Histology showed that these K5/HA-GαqQL mice developed skin carcinoma (middle lower panel). Immunofluorescence assays in frozen tissues show that K5/HA-GαqQL mice exhibit YAP (green) nuclear translocation, using Hoechst to stain nuclear DNA (blue) (right lower panels). See also Figure S2.
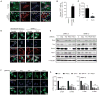
Figure 3. Trio and a network of Rho-GTPases mediate YAP activation in uveal melanoma cells harboring GNAQ mutations
(A) Immunofluorescence assays using frozen tissues from clinical uveal melanoma specimens (n = 6) showed HMB-45-positive cells (red) with nuclear YAP (green), using Hoeschst to stain nuclear DNA (blue), using as control HMB-45 staining to identify the resident melanocytes in normal tissues (n = 3). (B) Quantification of percent nuclear YAP positive cells in melanosome positive cells. (C) PLCi inhibits the hydrolysis of phosphoinositides in OMIM1.3 uveal melanoma cells as judged by PI turnover assays. (D) HEK293 cells transfected with GαqQL expression vectors and OMM1.3 uveal melanoma cells exhibited nuclear YAP by immunofluorescence (green), which was insensitive to PLC inhibition (PLCi), using Hoeschst and phalloidin to stain nuclear DNA (blue) and cytoplasmic polymerized actin (red), respectively. (E) Western blot analysis documents knock down using siRNAs in two uveal melanoma cell lines. (F) OMM1.3 uveal melanoma transfected with siRNA-control show cells with YAP (green) nuclear staining, while cells transfected with the indicated siRNAs show YAP mainly localized to the cytoplasm. Hoechst stains nuclear DNA (blue). (G) siRNAs knockdown of Gαq, Trio, RhoA or Rac1 diminish the expression of endogenous YAP-regulated genes (CTGF and CYR61) in OMM1.3 and OMM1.5 uveal melanoma cells (mean ± SEM, n = 3).
Figure 4. GNAQ oncogenic signaling induces YAP nuclear translocation and YAP-dependent transcription activation through Rho-GTPases and actin remodeling
(A) Western blots show expression of total and phosphorylated (serine 127) YAP and LATS1 and LATS2 in uveal and cutaneous melanoma cells, the latter expressing BRAF and NRAS oncogenes, as indicated, as well as in HEK293 cells expressing GαqQL or vector (v) as controls. (B and C) Cell lysates were subjected to immunoblotting with the indicated antibodies. Gels containing phos-tag were employed to assess YAP phosphorylation status. Dephosphorylated YAP was reflected by the faster migration of YAP. (B) Phosphorylated YAP (p-YAP and slower mobility forms) and dephosphorylated YAP in the cytosolic (Cyt.) and nuclear (Nucleus) fractions of HEK293 cells transfected with GαqQL or vector control. (C) Phosphorylated YAP (slower mobility forms) in uveal and cutaneous melanoma cells expressing the indicated oncogenes. (D) HEK293 cells were cotransfected with siRNA LATS1 and LATS2 or control and HA-GαqQL or vector DNAs, followed by the indicated Western blot analysis for HA-Gαq, LATS1, LATS2, p(127)-YAP, YAP and α-Tubulin as a loading control. (E) Similarly, cells were also transfected with Gal4-TEAD4, 5×UAS-Luc and Renilla-Luc DNAs, followed by luciferase assay (mean ± SEM, n = 3). (F) Cells were also studied by qPCR to assess the expression levels of YAP-regulated genes (CTGF and CYR61) (mean ± SEM, n = 3). (G) Levels of cofilin and phospho-cofilin (p-cofilin) in HEK293 cells expressing GαqQL or vector control, as well as in the indicated uveal and cutaneous melanoma cells. (H) Accumulation of phosphorylated cofilin in HEK293 cells expressing RhoAQL or Rac1QL. (I) Expression of YAP regulated genes (CTGF and CYR61) in HEK293 cells expressing RhoAQL or Rac1QL (mean ± SEM, n = 3). (J) OMM1.3 uveal melanoma cells treated with Y-27632 or Latrunculin A (Lat.A), following with Immunofluorescence assay, YAP (green), Hoechst stains nuclear DNA (blue) and phalloidin stains F-actin (violet). (K) Proportion of cells displaying preferential nuclear (N), nuclear and cytoplasmic (N/C), or cytoplasmic (C) YAP location (left panel; N=50–100 cells). (L) Y-27632 or Lat.A treatments were followed by Western blot analysis for p-cofilin, cofilin, p127-YAP, YAP, and α-Tubulin as a loading control. (M) Impact of Y-27632 and Lat.A treatments on the expression of endogenous YAP-regulated genes (CTGF and CYR61) in OMM1.3 and OMM1.5 uveal melanoma cells (mean ± SEM, n = 3). See also Figure S3.
Figure 5. Actin remodeling results in Hippo-independent activation of YAP downstream from GNAQ oncogenic signaling
(A) OMM1.3 and OMM1.5 cells were transfected with siRNAs for LATS1 and LATS2 and treated with control diluent or Y-27632 and Lat.A, followed by Western blot analysis for LATS1, LATS2, p-cofilin, cofilin, p127-YAP, YAP and α-Tubulin as a loading control. (B) Similarly, cells were also followed by qPCR to analyze the expression of YAP-regulated genes (CTGF and CYR61) (mean ± SEM, n = 3). (C) HEK293 cells were cotransfected with siRNA LATS1 and LATS2 and HA-GαqQL, and treated with Y-27632 or Lat.A followed by the indicated Western blot analysis for HA-GαqQL, LATS1, LATS2, p-cofilin, cofilin, p127-YAP, YAP and α-Tubulin as a loading control. (D) Cells were also followed by qPCR to assess the expression levels of YAP-regulated genes (CTGF and CYR61) (mean ± SEM, n = 3). (E) OMM1.3 cells expressing flag-tagged YAP treated with Lat.A or control, were lysed and followed by anti-flag and control (IgG) immunoprecipitation (IP) and Western blot analysis for flag-YAP, AMOT, LATS1, and 14-3-3 present in the immuneprecipitates, using the input lysate as control. (F) Anti-flag immunoprecipitates (IP) from HEK293 cells expressing flag-YAP were exposed to G-actin or F-actin, washed, and analyzed by Western blot for flag-YAP and associated endogenous AMOT. (G) OMM1.3 cells were transfected with siRNA for AMOT, followed by the indicated Western blot analysis for AMOT (recognizing both p130 and p80 forms) and α-Tubulin as a loading control. (H) OMM1.3 were transfected with siRNA AMOT or siRNA control, followed by Lat.A treatment or control, and the expression of YAP-regulated genes (CTGF and CYR61) was determined by qPCR. (I) Schematic representation of Hippo-dependent and –independent pathways resulting in YAP activation by the GNAQ oncogene in uveal melanoma. Gαq protein stimulates YAP through a RhoA and Rac1 regulated signaling circuitry initiated by the activation of TRIO, a Rho-GEF activating these GTPases, independently of the best-known stimulation of second messengers through PLC-β. In turn, RhoA activates ROCK and Rac1 stimulates PAK proteins, which converge in the activation of LIMK that phosphorylates and inactivates the actin severing protein cofilin, resulting in actin polymerization and F-actin accumulation (not depicted for simplicity, dotted line). F–actin may then bind AMOT, displacing YAP, which translocates to the nucleus and initiates gene expression. Free YAP can also bind to LATS, which phosphorylates and inactivates YAP upon the cytosolic sequestration of phospho-YAP by 14-3-3 proteins or by promoting its proteosomal degradation (the latter not depicted), as part of a canonical Hippo-dependent pathway restraining YAP function. How Rho GTPases regulate LATS function is not fully understood (dotted line). It is expected that in the presence of GNAQ oncogenes, LATS reduced activity acts in a coordinated function with the likely dominant F-actin-mediated stimulation of YAP to promote YAP stabilization and nuclear translocation, ultimately resulting in the expression of YAP-regulated growth-promoting genes. See text for details.
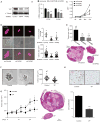
Figure 6. YAP represents a therapeutic target in uveal melanoma
(A) Western blot show YAP knockdown by doxycycline inducible shRNAs (YAP#1 and YAP#2) in OMM1.3 uveal melanoma cells. (B) Impact of shRNAs knocking down YAP on the expression of endogenous YAP-regulated genes (CTGF and CYR61) in OMM1.3 uveal melanoma cells (mean ± SEM, n = 5). (C) Effect of shRNAs knock down of YAP in OMM1.3 uveal melanoma cells proliferation (mean ± SEM, n = 3). (D) OMM1.3 uveal melanoma cells colony formation in soft agar after shRNA-mediated knockdown of YAP. shRNA positive cells (control and YAP#1 and YAP#2) expressed Tomato (red) (left panel), and were counted (right upper panel) (mean ± SEM, n = 10) and size measured (right lower panel) with Image-J (mean ± SEM, n = 20–100 colonies). (E) OMM1.3 uveal melanoma formation in vivo in cells expressing control and YAP shRNAs. Tumor size at the end of the study was measured (mean ± SEM, n = 6) (upper panel); H&E-stained sections of representative tumors from each group are shown (lower panel). (F) Soft agar assays show the effect of VP treatment on OMM1.3 uveal melanoma cells colony formation ability (left panel) and colony size (mean ± SEM, n = 20–50 colonies) (right panel). (G) Effect of VP on OMM1.3 uveal melanoma cells growth in vivo. Tumor size was measured every four days after the initiation of the VP treatment and control (mean ± SEM, n = 6) (n = numbers of tumors analyzed) (left panel). H&E-stained sections of representative tumors from each group (right panels). (H) Effect of VP treatment on OMM1.3 uveal melanoma cells
Comment in
- GNAQ/11 mutations in uveal melanoma: is YAP the key to targeted therapy?
Field MG, Harbour JW. Field MG, et al. Cancer Cell. 2014 Jun 16;25(6):714-5. doi: 10.1016/j.ccr.2014.05.028. Cancer Cell. 2014. PMID: 24937456 Free PMC article.
Similar articles
- GNAQ/11 mutations in uveal melanoma: is YAP the key to targeted therapy?
Field MG, Harbour JW. Field MG, et al. Cancer Cell. 2014 Jun 16;25(6):714-5. doi: 10.1016/j.ccr.2014.05.028. Cancer Cell. 2014. PMID: 24937456 Free PMC article. - Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP.
Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, Zhao J, Chen X, Zhang L, Wang CY, Bastian BC, Zhang K, Guan KL. Yu FX, et al. Cancer Cell. 2014 Jun 16;25(6):822-30. doi: 10.1016/j.ccr.2014.04.017. Epub 2014 May 29. Cancer Cell. 2014. PMID: 24882516 Free PMC article. - A Platform of Synthetic Lethal Gene Interaction Networks Reveals that the GNAQ Uveal Melanoma Oncogene Controls the Hippo Pathway through FAK.
Feng X, Arang N, Rigiracciolo DC, Lee JS, Yeerna H, Wang Z, Lubrano S, Kishore A, Pachter JA, König GM, Maggiolini M, Kostenis E, Schlaepfer DD, Tamayo P, Chen Q, Ruppin E, Gutkind JS. Feng X, et al. Cancer Cell. 2019 Mar 18;35(3):457-472.e5. doi: 10.1016/j.ccell.2019.01.009. Epub 2019 Feb 14. Cancer Cell. 2019. PMID: 30773340 Free PMC article. - GNAQ and GNA11 mutations in uveal melanoma.
Shoushtari AN, Carvajal RD. Shoushtari AN, et al. Melanoma Res. 2014 Dec;24(6):525-34. doi: 10.1097/CMR.0000000000000121. Melanoma Res. 2014. PMID: 25304237 Review. - Research in practice: Therapeutic targeting of oncogenic GNAQ mutations in uveal melanoma.
Gaffal E. Gaffal E. J Dtsch Dermatol Ges. 2020 Nov;18(11):1245-1248. doi: 10.1111/ddg.14288. Epub 2020 Sep 21. J Dtsch Dermatol Ges. 2020. PMID: 32954611 Review.
Cited by
- Selective targeting of KRAS-driven lung tumorigenesis via unresolved ER stress.
Shimomura I, Watanabe N, Yamamoto T, Kumazaki M, Tada Y, Tatsumi K, Ochiya T, Yamamoto Y. Shimomura I, et al. JCI Insight. 2021 Apr 8;6(7):e137876. doi: 10.1172/jci.insight.137876. JCI Insight. 2021. PMID: 33830081 Free PMC article. - G Protein-Coupled Receptors in Cancer.
Bar-Shavit R, Maoz M, Kancharla A, Nag JK, Agranovich D, Grisaru-Granovsky S, Uziely B. Bar-Shavit R, et al. Int J Mol Sci. 2016 Aug 12;17(8):1320. doi: 10.3390/ijms17081320. Int J Mol Sci. 2016. PMID: 27529230 Free PMC article. Review. - Ephexin3/ARHGEF5 Together with Cell Migration Signaling Partners within the Tumor Microenvironment Define Prognostic Transcriptional Signatures in Multiple Cancer Types.
Juan-Guadarrama DG, Beltrán-Navarro YM, Reyes-Cruz G, Vázquez-Prado J. Juan-Guadarrama DG, et al. Int J Mol Sci. 2023 Nov 17;24(22):16427. doi: 10.3390/ijms242216427. Int J Mol Sci. 2023. PMID: 38003617 Free PMC article. - Biological characteristics and clinical management of uveal and conjunctival melanoma.
Kaštelan S, Pavičić AD, Pašalić D, Nikuševa-Martić T, Čanović S, Kovačević P, Konjevoda S. Kaštelan S, et al. Oncol Res. 2024 Jul 17;32(8):1265-1285. doi: 10.32604/or.2024.048437. eCollection 2024. Oncol Res. 2024. PMID: 39055896 Free PMC article. Review. - Targeting the YAP/TAZ Pathway in Uveal and Conjunctival Melanoma With Verteporfin.
Brouwer NJ, Konstantinou EK, Gragoudas ES, Marinkovic M, Luyten GPM, Kim IK, Jager MJ, Vavvas DG. Brouwer NJ, et al. Invest Ophthalmol Vis Sci. 2021 Apr 1;62(4):3. doi: 10.1167/iovs.62.4.3. Invest Ophthalmol Vis Sci. 2021. PMID: 33798262 Free PMC article.
References
- Adler JJ, Johnson DE, Heller BL, Bringman LR, Ranahan WP, Conwell MD, Sun Y, Hudmon A, Wells CD. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of Angiomotin by the LATS1/2 protein kinases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17368–17373. - PMC - PubMed
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell. 2013;154:1047–1059. - PubMed
- Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. - PubMed
- Barbazetto IA, Lee TC, Rollins IS, Chang S, Abramson DH. Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol. 2003;135:898–899. - PubMed
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the G(i) subfamily of G protein alpha subunits. Cell. 1996;86:445–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 EY021189/EY/NEI NIH HHS/United States
- K22 CA163799/CA/NCI NIH HHS/United States
- ZIA DE000551-22/ImNIH/Intramural NIH HHS/United States
- ZIA DE000558-22/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous