mTOR inhibition and levels of the DNA repair protein MGMT in T98G glioblastoma cells - PubMed (original) (raw)
mTOR inhibition and levels of the DNA repair protein MGMT in T98G glioblastoma cells
Sarah Smalley et al. Mol Cancer. 2014.
Abstract
Background: Glioblastoma multiforme (GBM), the most common and most aggressive type of primary adult brain tumour, responds poorly to conventional treatment. Temozolomide (TMZ) chemotherapy remains the most commonly used treatment, despite a large proportion of tumours displaying TMZ resistance. 60% of GBM tumours have unmethylated MGMT promoter regions, resulting in an overexpression of the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT), which is responsible for tumour resistance to TMZ chemotherapy. Tumours also often exhibit hyperactive PI3-kinase/mTOR signalling, which enables them to resynthesise proteins quickly. Since MGMT is a suicide protein that is degraded upon binding to and repairing TMZ-induced O6-methylguanine adducts, it has been hypothesized that inhibition of translation via the mTOR signalling pathway could generate a tumour-specific reduction in MGMT protein and increase TMZ sensitivity.
Methods: MGMT was monitored at the post-transcriptional, translational and protein levels, to determine what effect mTOR inhibition was having on MGMT protein expression in vitro.
Results: We show that inhibiting mTOR signalling is indeed associated with acute inhibition of protein synthesis. Western blots show that despite this, relative to loading control proteins, steady state levels of MGMT protein increased and MGMT mRNA was retained in heavy polysomes. Whilst TMZ treatment resulted in maintained MGMT protein levels, concomitant treatment of T98G cells with TMZ and KU0063794 resulted in increased MGMT protein levels without changes in total mRNA levels.
Conclusions: These in vitro data suggest that, counterintuitively, mTOR inhibition may not be a useful adjunct to TMZ therapy and that more investigation is needed before applying mTOR inhibitors in a clinical setting.
Figures
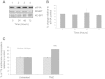
Figure 1
TMZ treatment does not affect steady state levels of MGMT protein. T98G cells were incubated in the absence (lane 1) or presence of TMZ for 24 hours (lane 2), 48 hours (lanes 3) or 72 hours (lane 4). A. Proteins were visualised by Western blotting using the antiserum indicated. B. MGMT protein levels in (A) were quantified and expressed relative to the eIF4A loading control. Error bars are the SE (n = 3). Confidence limits were set: *p = <0.2, **p = <0.05 ***p = <0.005. C. Cells were incubated with [35S] methionine, as described in Materials and Methods. Incorporation of radioactive methionine into protein was determined as cpm/μg protein; results are presented as a % of methionine incorporated in to cells incubated in the absence of TMZ. Error bars are the S.D (n = 3).
Figure 2
TMZ combined with KU0063794 decreases MGMT protein levels compared to treatments with KU0063794 alone, but an increase in overall MGMT protein levels is still observed. T98G cells were incubated in the absence (lane 1) or presence of TMZ and KU0063794 for 12 hours (lane 2), 24 hours (lanes 3), 48 hours (lane 4) or 72 hours (lane 5). A Proteins were visualised by Western blotting using the antiserum indicated. B. MGMT protein levels in (A) were quantified and expressed relative to the α tubulin loading control. Error bars are the SE (n = 3). Confidence limits were set: *p = <0.2, **p = <0.05 ***p = <0.005. C. Cells were incubated in the absence or presence of KU0063794, TMZ and KU0063794 or TMZ alone. Cells were incubated with [35S] methionine, as described in Materials and Methods. Incorporation of radioactive methionine into protein was determined as cpm/μg protein; results are presented as a% of methionine incorporated in to cells incubated in the absence of any treatments. Error bars are the S.D (n = 3).
Figure 3
KU0063794 inhibits protein translation via mTOR kinase inhibition in T98G cells but MGMT protein levels are increased. T98G cells were incubated in the absence (lane 1) or presence of KU0063794 for 12 hours (lane 2), 24 hours (lanes 3), 48 hours (lane 4) or 72 hours (lane 5). A. Western blotting was carried out as described in Materials and Methods B. Aliquots of protein extract were subjected to m7GTP-Sepharose affinity chromatography as described in Materials and Methods. Western blotting was carried out. All lanes were resolved on the same gel. C. T98G cells were incubated in the absence (lane 1) or presence of KU0063794 for 12 hours (lane 2), 24 hours (lanes 3), 48 hours (lane 4) or 72 hours (lane 5). Proteins were visualised by Western blotting using the antiserum indicated. D. MGMT protein levels in (C) were quantified and expressed relative to the α tubulin loading control. Error bars are the SE (n = 3). Confidence limits were set: *p = <0.2, **p = <0.05 ***p = <0.005. E. Cells were incubated in the absence or presence of KU0063794 as indicated. Mitochondrial activity (a measure of cell viability) was assessed using an MTS assay as described in Materials and Methods and is expressed relative to untreated cells (set at 100%). Error bars are the S.D (n = 3).
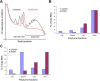
Figure 4
KU0063794 inhibits global protein translation, while increasing translation of the MGMT protein. Cells were incubated in the absence or presence of KU0063794. A. Cell lysates were prepared, polysomes fractionated using sucrose density gradients. The UV profile from this analysis is shown here and the fractionation of 40S, 560S, 80S ribosomes and polysomes is indicated. mRNA from pooled fractions was subsequently extracted as described in the Materials and Methods. For each gradient, qRT-PCR was used to determine levels of GAPDH and MGMT mRNA distribution across each fraction (free represents RNA and associated material at the top of the gradient and subunits show 40S/60S/80S-bound RNA), with the total mRNA in each gradient set at 100%. Cells were incubated for 24 hours in the absence (B) or presence of KU0063794 (C).
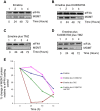
Figure 5
Inhibition of mTOR signalling does not affect the half- life of MGMT protein in T98G cells but stabilises MGMT in the presence of TMZ. T98G cells were incubated in the presence of emetine alone (A) or emetine and KU0063794 (B), emetine and TMZ (C) or emetine, KU0063794 and TMZ (D) for the times indicated. Western blotting was carried out as described in Materials and Methods and the antiserum used indicated. E. Western blots from the representative data shown were used to quantify MGMT protein levels using eIF4A as a loading control, as described above. MGMT protein levels are expressed relative to those found in untreated cells (set at 100%).
Similar articles
- Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients.
Agnihotri S, Gajadhar AS, Ternamian C, Gorlia T, Diefes KL, Mischel PS, Kelly J, McGown G, Thorncroft M, Carlson BL, Sarkaria JN, Margison GP, Aldape K, Hawkins C, Hegi M, Guha A. Agnihotri S, et al. J Clin Invest. 2012 Jan;122(1):253-66. doi: 10.1172/JCI59334. Epub 2011 Dec 12. J Clin Invest. 2012. PMID: 22156195 Free PMC article. - PARP-mediated PARylation of MGMT is critical to promote repair of temozolomide-induced O6-methylguanine DNA damage in glioblastoma.
Wu S, Li X, Gao F, de Groot JF, Koul D, Yung WKA. Wu S, et al. Neuro Oncol. 2021 Jun 1;23(6):920-931. doi: 10.1093/neuonc/noab003. Neuro Oncol. 2021. PMID: 33433610 Free PMC article. - Optimizing glioblastoma temozolomide chemotherapy employing lentiviral-based anti-MGMT shRNA technology.
Viel T, Monfared P, Schelhaas S, Fricke IB, Kuhlmann MT, Fraefel C, Jacobs AH. Viel T, et al. Mol Ther. 2013 Mar;21(3):570-9. doi: 10.1038/mt.2012.278. Epub 2013 Jan 15. Mol Ther. 2013. PMID: 23319055 Free PMC article. - A novel approach to overcome temozolomide resistance in glioma and melanoma: Inactivation of MGMT by gene therapy.
Jiang G, Wei ZP, Pei DS, Xin Y, Liu YQ, Zheng JN. Jiang G, et al. Biochem Biophys Res Commun. 2011 Mar 18;406(3):311-4. doi: 10.1016/j.bbrc.2011.02.042. Epub 2011 Feb 15. Biochem Biophys Res Commun. 2011. PMID: 21329652 Review. - Regulation of expression of O6-methylguanine-DNA methyltransferase and the treatment of glioblastoma (Review).
Cabrini G, Fabbri E, Lo Nigro C, Dechecchi MC, Gambari R. Cabrini G, et al. Int J Oncol. 2015 Aug;47(2):417-28. doi: 10.3892/ijo.2015.3026. Epub 2015 May 29. Int J Oncol. 2015. PMID: 26035292 Free PMC article. Review.
Cited by
- Iloperidone and Temozolomide Synergistically Inhibit Growth, Migration and Enhance Apoptosis in Glioblastoma Cells.
Mubeen S, Raza I, Ujjan B, Wasim B, Khan L, Naeem N, Enam SA, Hanif F. Mubeen S, et al. Biomedicines. 2024 May 21;12(6):1134. doi: 10.3390/biomedicines12061134. Biomedicines. 2024. PMID: 38927341 Free PMC article. - Inhibitory effect of temozolomide on apoptosis induction of cinnamaldehyde in human glioblastoma multiforme T98G cell line.
Abband H, Dabirian S, Jafari A, Nasiri M, Nasiri E. Abband H, et al. Anat Cell Biol. 2024 Mar 31;57(1):85-96. doi: 10.5115/acb.23.159. Epub 2023 Nov 22. Anat Cell Biol. 2024. PMID: 37994040 Free PMC article. - MGMT inhibition in ER positive breast cancer leads to CDC2, TOP2A, AURKB, CDC20, KIF20A, Cyclin A2, Cyclin B2, Cyclin D1, ERα and Survivin inhibition and enhances response to temozolomide.
Bobustuc GC, Kassam AB, Rovin RA, Jeudy S, Smith JS, Isley B, Singh M, Paranjpe A, Srivenugopal KS, Konduri SD. Bobustuc GC, et al. Oncotarget. 2018 Jul 3;9(51):29727-29742. doi: 10.18632/oncotarget.25696. eCollection 2018 Jul 3. Oncotarget. 2018. PMID: 30038716 Free PMC article. - Temozolomide resistance and tumor recurrence: Halting the Hedgehog.
Munoz JL, Rodriguez-Cruz V, Walker ND, Greco SJ, Rameshwar P. Munoz JL, et al. Cancer Cell Microenviron. 2015;2(2):e747. doi: 10.14800/ccm.747. Epub 2015 May 7. Cancer Cell Microenviron. 2015. PMID: 27158638 Free PMC article. - mTOR regulates the expression of DNA damage response enzymes in long-lived Snell dwarf, GHRKO, and PAPPA-KO mice.
Dominick G, Bowman J, Li X, Miller RA, Garcia GG. Dominick G, et al. Aging Cell. 2017 Feb;16(1):52-60. doi: 10.1111/acel.12525. Epub 2016 Sep 13. Aging Cell. 2017. PMID: 27618784 Free PMC article.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. Massachusetts Med Soc. 2005;352:987–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous