The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation - PubMed (original) (raw)
doi: 10.1038/ni.2913. Epub 2014 Jun 22.
Lukas Bossaller 2, Dominic De Nardo 1, Jacqueline M Ratter 1, Andrea Stutz 1, Gudrun Engels 1, Christoph Brenker 3, Mark Nordhoff 3, Sandra R Mirandola 3, Ashraf Al-Amoudi 3, Matthew S Mangan 4, Sebastian Zimmer 5, Brian G Monks 6, Martin Fricke 7, Reinhold E Schmidt 7, Terje Espevik 8, Bernadette Jones 9, Andrew G Jarnicki 9, Philip M Hansbro 9, Patricia Busto 10, Ann Marshak-Rothstein 10, Simone Hornemann 11, Adriano Aguzzi 11, Wolfgang Kastenmüller 12, Eicke Latz 13
Affiliations
- PMID: 24952505
- PMCID: PMC4116676
- DOI: 10.1038/ni.2913
The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation
Bernardo S Franklin et al. Nat Immunol. 2014 Aug.
Abstract
Microbes or danger signals trigger inflammasome sensors, which induce polymerization of the adaptor ASC and the assembly of ASC specks. ASC specks recruit and activate caspase-1, which induces maturation of the cytokine interleukin 1β (IL-1β) and pyroptotic cell death. Here we found that after pyroptosis, ASC specks accumulated in the extracellular space, where they promoted further maturation of IL-1β. In addition, phagocytosis of ASC specks by macrophages induced lysosomal damage and nucleation of soluble ASC, as well as activation of IL-1β in recipient cells. ASC specks appeared in bodily fluids from inflamed tissues, and autoantibodies to ASC specks developed in patients and mice with autoimmune pathologies. Together these findings reveal extracellular functions of ASC specks and a previously unknown form of cell-to-cell communication.
Figures
Figure 1
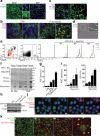
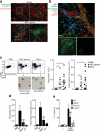
ASC specks accumulate in the extracellular space after inflammasome activation. (a) Confocal imaging of untreated or ATP-activated (5 mM, 40 min) ASC-mCerulean expressing iMøs, Scale bar: 22 μm. (b) Ex-vivo immunostaining of the subcapsular sinus from permeabilized sections of popliteal LNs from mice 4 h after injection with PBS, or P. aeruginosa. Scale bar: 15 μm and 19 μm. (c) Confocal imaging of ASC-mCerulean iMøs treated as in (a). Arrows indicate extracellular ASC specks. Scale bars: 4.4 μm and 3 μm. (d) Fluorescence imaging of LPS-primed (1 μg/ml) and nigericin-activated (10 μM) ASC-mCerulean expressing THP-1 monocytes. Arrows indicate extracellular ASC specks. Scale bar: 10 μm. (e) Flow cytometry and (i) quantification of extracellular specks in cell-free supernatants of ASC-mCerulean THP-1s treated as in d. (f) Immunoblot of DSS cross-linked endogenous ASC in cell pellets, or 5 μm filtrates of cell-free supernatants from WT THP-1 monocytes treated as in d. (g) Quantification of extracellular specks and (h) immunoblot of ASC in LPS-primed (250 ng/ml, 3h) iMøs treated as indicated. (i) Assessment of LDH release in cell-free supernatants of ASC-mCerulean THP-1s treated as in d, calculated from cells treated with 1% Triton X100. (j) Confocal time lapse of ASC-mCerulean THP-1 monocytes treated as in d in the presence of propidium iodide (1 μg/ml). Scale bars 8 μm, time (min). (k) Confocal time lapse of ATP-activated ASC-mCerulean iMøs in presence of directly conjugated anti-GFP Alexa 555 mAb (1 μg). Scale bars 33.0 μm, time (min). Data are representative of three (a,c-e,i) or two independent experiments (b,f-h,j,k); (e)Technical triplicates (colored lines) from one representative of three independent experiments; (i) combine data from two independent experiments (mean + SD of the number of specks per μl of acquired sample); g, mean + SD of triplicates from a representative of two independent experiments.
Figure 2
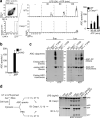
Extracellular specks remain active in the extracellular space. Flow cytometry (a) and quantification of extracellular ASC specks (b) in cell-free supernatants of LPS-primed ATP activated ASC-mCerulean expressing iMøs from either Casp1−/− or WT mice, treated or not with 10 μM of YVAD for 30 min. (c) Immunoblot of DSS cross-linked-ASC in 5 μm filtered cell-free supernatants and whole cell lysates of WT or Casp1−/− iMøs treated as in a. (d) Immunoblot of IL-1β and caspase-1 in cytosols prepared from untreated or LPS-primed Pycard−/− iMøs and left untreated (None) or incubated with _in vitro-_assembled ASC specks. Data are representative of four (a-c) or two (d) independent experiments.
Figure 3
Extracellular specks represent a cell-derived danger signal. (a) Confocal imaging of iMøs incubated with _in vitro_-assembled ASC-mCerulean specks for 2 hours. Scale bar 9 μm. (b) Immunoblot of ASC-mCerulean (anti-GFP) in lysates of BMDMs incubated with ASC-mCerulean specks. Non-phagocyted specks were washed away. (c) Confocal imaging of iMøs incubated with Dextran-A488 (20 μg/ml) alone or together with the lysosomal damaging LeuLeu-O-Me (0.5 μM), or ASC-mCerulean specks (100 μg/ml) for 4 hours. Scale bars: 7.0 μm top left, 4.9 μm top right, 6.0 μm bottom left, and 2.9 μm bottom right. (d) Average size of lysosomes in cells treated as indicated. (e) Percentage of recipient ASC-mCherry cells containing ASC-mCherry specks after incubation with silica crystals (100 μg/ml), nigericin (10 μM) or ASC-mCerulean specks (100 μg/ml). (f-g) ELISA of IL-1β in supernatants of LPS-primed (200 ng/ml, 2 h) BMDMs that were either left untreated or stimulated with cholesterol crystals (250 μg/ml), ASC specks, or a mock preparation from Pycard−/− iMøs (200 μg/ml). Data are representative from three independent experiments (a-e); d, mean + SD of combined data from two independent experiments, in which at least 5 fields/condition were analyzed (Mann-Whitney test, **P < 0.0057); e, combined data from multiple fields (n = 8) from two independent experiments (mean ± SD, Mann-Whitney test, *P < 0.05, **P < 0.006); f, Mean + s.e.m. of combined data from four independent experiments; g, mean + SD of combined data from two independent experiments.
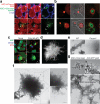
Figure 4
ASC has prionoid features. Confocal imaging of ASC-mCherry expressing iMøs incubated overnight (a), or 36 hours (b) with _in vitro_-assembled ASC-mCerulean specks. Open triangles indicate ASC-mCerulean specks that recruited ASC-mCherry and closed triangle indicate ASC-mCerulan specks that have not recruited ASC-mCherry. Scale bars: 2.6 μm. (c) Confocal imaging of untreated or LPS-primed, poly dAdT-activated ASC-mCerulean expressing iMøs. Scale bar: 4.9 μm. (d) Stimulated emission depletion (STED) microscopy of ASC specks purified from ASC-mCerulean expessing iMøs stimulated with ATP and stained with anti-GFP and Atto 647N-conjugated secondary Abs. Scale bar: 0.5 μm. (e) Electron microscopy (EM) of ASC specks prepared from WT or mock preparations from Pycard−/− iMøs. Scale bars: 100 nm. (f) EM of _in vitro-_assembled ASC-mCerulean specks. Scale bars: 0.5 μm. (g) EM of ASC-mCerulean specks isolated from LPS-primed, nigericin activated iMøs stained with anti-GFP Abs directly conjugated to 10 nm gold nanoparticles. Data (a-g) are representative of at least two independent experiments.
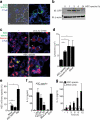
Figure 5
ASC specks propagate inflammation in vivo. (a) Confocal imaging of whole mount ear skin of LysM_gfp/gfp_ mice 4 h after subdermal injection of ASC-mCerulean specks (5 μg in 10 μl of PBS) or 10 μl of PE calibrate beads. (b) Confocal microscopy imaging of whole mount ear skin of WT mice 4 h after subdermal injection of ASC-mCerulean specks. Neutrophils (anti-Ly6G), endothelial/stomal cells (ECs) (anti-CD31). (c) Gating strategy and quantification of peritoneal lavage cells CD11b+Gr-1+F4/80− neutrophils and CD11b+Ly6C+Ly6G−F4/80− inflammatory monocytes in the peritoneum of WT mice 16 h after intraperitoneal injection of silica crystals (250 μg) or ASC specks (200 μg). (d) Quantification as in (e) of peritoneal lavage cells in WT mice or Nlrp3−/− and Il1r−/− mice injected with ASC specks (20 μg). (e) ELISA of IL-1β in supernatants of LPS-primed WT, Nlrp3−/−, Asc−/− or Casp1−/− iMøs that were left untreated (None) or stimulated with _in vitro_-assembled ASC specks (100 μg/ml). Representative of two (a-b) independent experiments; c, mean ± s.e.m. of pooled data from three independent experiments. Each symbol represents one mouse (n = 8 per group). Neutrophils: **P = 0.0006, Mann Whitney test; **P = 0.0018, unpaired two-tailed Student's t test. Inflammatory monocytes: **P = 0.0021, Mann Whitney test; **P = 0.0023, Mann Whitney test). d, mean ± SD from combined data from two independent experiments. PBS (n = 4), WT (n = 15), Nlrp3−/− (n = 8), Il1r−/− (n = 4). Neutrophils: **P = 0.0081, Inflammatory Monocytes: **P = 0.0040 , Mann Whitney test; (e) Mean ± s.e.m. of combined data from three independent experiments, Mann Whitney test, p < 0.05.
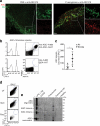
Figure 6
Extracellular ASC specks are formed in vivo and accumulate during human chronic inflammatory disease. (a) Confocal imaging of subcapsular sinus macrophages of popliteal draining LNs from P. aeruginosa infected mice injected 4 h later via the same route with 4 μg of PE-labeled anti-ASC Abs. Scale bars: 53 μm left panels 37 μm right panels, 9 μm inserts. (b) Flow cytometry of ASC-mCerulean specks pre-incubated with two monoclonal anti-ASC Abs (top histograms) or IgG1 isotype controls (bottom histograms) directly labeled with A488 and A647 fluorophores. Amount of specks per μl of sample was determined by subtracting A488+A647+ events in anti-ASC stained sample from those in IgG1 control stained samples. (c) Adjusted quantification of A488+A647+ ASC specks in cell-free BALF from WT BALB/c mice exposed to cigarette smoke or normal air for 8 weeks. (d) Flow cytometry of BALF samples from patients with COPD (5 ml/patient) before and after removal of dead cells by centrifugation (400 x g, 5 min) and size filtration (5 μm). (e) Immunoblotting for ASC (right) of cell-free filtered BALF samples from patients with COPD (n = 4), Pneumonia (n = 4), Pulmunary hypertension (n =2) or healthy donors (n =2) after chemical crosslinking with 1 mM of DSS. Cell-free sups from untreated (–) or LPS-primed, nigericin-activated THP-1s were used as controls. Data show representative images (a) flow cytometry (b, d), and immunoblotting (e) analysis from one out of two independent experiments; (c) cumulative from one experiment; each symbol represents an individual mouse; horizontal and vertical lines indicate mean + SD, *P = 0.0317, Mann-Whitney test.
Figure 7
(a) Quantification of peritoneal neutrophils and inflammatory monocytes in peritoneal lavage from C57BL/6 mice intravenously injected with anti-ASC pAb (100 μg) or purified rabbit IgG (100 μg) and intraperitoneally injected 2 h later with silica crystals (250 μg). (b) Fluorescence microscopy of BMDMs incubated with untreated or antibody coated (1 μg) ASC-mCerulean specks. Scale bars: 90 μm. (c) Percentages of macrophages with phagocytosed ASC-mCerulean specks. (d) Quantification of mCerulean positive lysosomes. (e) ELISA of IL-1β in supernatants of LPS-primed BMDMs left untreated, or stimulated with _in vitro-_assembled ASC specks pre-coated with 1 μg of anti-ASC Abs. (f) Flow cytometry screening method and median fluorescence intensity (graph) for the reactivity of sera from healthy donors (n = 7) or ANA+ autoimmune patients (n = 80) to ASC-mCerulean specks. (g) ELISA of IL-1β in supernatants of LPS-primed BMDMs left untreated, or stimulated with _in vitro_-assembled ASC specks coated with anti-ASC pAb or pooled (n = 4) sera from pristane-induced SLE or control mice. Data are representative of three (b-d) or two independent experiments (a,f,g). a culmulative from one experiment (mean + SD) PBS + IgG (n = 3), PBS + anti-ASC (n = 3), silica + IgG (n = 4), silica + anti-ASC (n = 7). Neutrophils: **P = 0.0061, Mann Whitney test; Inflammatory monocytes: **P = 0.0028, unpaired two-tailed Student's t test; Culmulative from one experiment in which 3 fields/condition (c) and 20 cells/condition (d) were analyzed. Each symbol represents one cell (mean ± SD); e, Combined data from two independent experiments performed in triplicates (Mean + s.em); g, mean + SD of one representative out of two independent experiments.
Similar articles
- Unknown/enigmatic functions of extracellular ASC.
de Souza JG, Starobinas N, Ibañez OCM. de Souza JG, et al. Immunology. 2021 Aug;163(4):377-388. doi: 10.1111/imm.13375. Epub 2021 Jun 22. Immunology. 2021. PMID: 34042182 Free PMC article. Review. - The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response.
Baroja-Mazo A, Martín-Sánchez F, Gomez AI, Martínez CM, Amores-Iniesta J, Compan V, Barberà-Cremades M, Yagüe J, Ruiz-Ortiz E, Antón J, Buján S, Couillin I, Brough D, Arostegui JI, Pelegrín P. Baroja-Mazo A, et al. Nat Immunol. 2014 Aug;15(8):738-48. doi: 10.1038/ni.2919. Epub 2014 Jun 22. Nat Immunol. 2014. PMID: 24952504 - A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages.
Zheng Y, Lilo S, Brodsky IE, Zhang Y, Medzhitov R, Marcu KB, Bliska JB. Zheng Y, et al. PLoS Pathog. 2011 Apr;7(4):e1002026. doi: 10.1371/journal.ppat.1002026. Epub 2011 Apr 21. PLoS Pathog. 2011. PMID: 21533069 Free PMC article. - Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production.
Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE. Man SM, et al. J Immunol. 2013 Nov 15;191(10):5239-46. doi: 10.4049/jimmunol.1301581. Epub 2013 Oct 11. J Immunol. 2013. PMID: 24123685 Free PMC article. - Caspase-1-induced pyroptotic cell death.
Miao EA, Rajan JV, Aderem A. Miao EA, et al. Immunol Rev. 2011 Sep;243(1):206-14. doi: 10.1111/j.1600-065X.2011.01044.x. Immunol Rev. 2011. PMID: 21884178 Free PMC article. Review.
Cited by
- The inflammasome pathway in stable COPD and acute exacerbations.
Faner R, Sobradillo P, Noguera A, Gomez C, Cruz T, López-Giraldo A, Ballester E, Soler N, Arostegui JI, Pelegrín P, Rodriguez-Roisin R, Yagüe J, Cosio BG, Juan M, Agustí A. Faner R, et al. ERJ Open Res. 2016 Jul 11;2(3):00002-2016. doi: 10.1183/23120541.00002-2016. eCollection 2016 Jul. ERJ Open Res. 2016. PMID: 27730204 Free PMC article. - Unknown/enigmatic functions of extracellular ASC.
de Souza JG, Starobinas N, Ibañez OCM. de Souza JG, et al. Immunology. 2021 Aug;163(4):377-388. doi: 10.1111/imm.13375. Epub 2021 Jun 22. Immunology. 2021. PMID: 34042182 Free PMC article. Review. - Assembly and regulation of ASC specks.
Hoss F, Rodriguez-Alcazar JF, Latz E. Hoss F, et al. Cell Mol Life Sci. 2017 Apr;74(7):1211-1229. doi: 10.1007/s00018-016-2396-6. Epub 2016 Oct 19. Cell Mol Life Sci. 2017. PMID: 27761594 Free PMC article. Review. - Biological and clinical roles of IL-18 in inflammatory diseases.
Landy E, Carol H, Ring A, Canna S. Landy E, et al. Nat Rev Rheumatol. 2024 Jan;20(1):33-47. doi: 10.1038/s41584-023-01053-w. Epub 2023 Dec 11. Nat Rev Rheumatol. 2024. PMID: 38081945 Review. - The alarmin interleukin-1α causes preterm birth through the NLRP3 inflammasome.
Motomura K, Romero R, Garcia-Flores V, Leng Y, Xu Y, Galaz J, Slutsky R, Levenson D, Gomez-Lopez N. Motomura K, et al. Mol Hum Reprod. 2020 Sep 1;26(9):712-726. doi: 10.1093/molehr/gaaa054. Mol Hum Reprod. 2020. PMID: 32647859 Free PMC article.
References
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. - PubMed
- Masumoto J, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274:33835–33838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL112661/HL/NHLBI NIH HHS/United States
- R01-HL093262/HL/NHLBI NIH HHS/United States
- R01-AI083713/AI/NIAID NIH HHS/United States
- R01 HL112661/HL/NHLBI NIH HHS/United States
- R01 HL093262/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous