The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase - PubMed (original) (raw)
The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase
Un Na et al. Cell Metab. 2014.
Abstract
Disorders arising from impaired assembly of succinate dehydrogenase (SDH) result in a myriad of pathologies, consistent with its unique role in linking the citric acid cycle and electron transport chain. In spite of this critical function, however, only a few factors are known to be required for SDH assembly and function. We show here that two factors, Sdh6 (SDHAF1) and Sdh7 (SDHAF3), mediate maturation of the FeS cluster SDH subunit (Sdh2/SDHB). Yeast and Drosophila lacking SDHAF3 are impaired in SDH activity with reduced levels of Sdh2. Drosophila lacking the Sdh7 ortholog SDHAF3 are hypersensitive to oxidative stress and exhibit muscular and neuronal dysfunction. Yeast studies revealed that Sdh6 and Sdh7 act together to promote Sdh2 maturation by binding to a Sdh1/Sdh2 intermediate, protecting it from the deleterious effects of oxidants. These studies in yeast and Drosophila raise the possibility that SDHAF3 mutations may be associated with idiopathic SDH-associated diseases.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
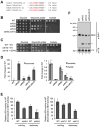
Figure 1. Succinate dehydrogenase deficiency in cells lacking two LYR motif family proteins, Sdh6 and Sdh7
(A) L-X-L/A-Y-R-X-X-L/I-R/K motif conserved in four LYR motif family proteins in yeast. Isd11, a chaperone required for cysteine desulfurase activity in FeS biogenesis pathway (Adam et al., 2006); Mzm1, a protein facilitating the Rieske FeS protein insertion into bc1 (Cui et al., 2012); Sdh6 (SDHAF1); and Sdh7. (B) 10-fold serial dilutions of cells starting from OD600=0.5 were spotted on synthetic complete (SC) media containing different carbon sources as indicated, and incubated at 30°C. (C) 10-fold serial dilutions of cells were spotted on SC media lacking uracil and incubated at 30°C. EV, empty vector (D) Metabolites extracted from cells cultured in synthetic minimal media containing 2% raffinose / 0.2% glucose were analyzed using GC-MS. Cells were harvested at OD600 = 2. Relative levels of metabolites to WT are represented as mean ± SEM (N ≥ 4 biological replicates; *p<0.05; **p<0.005). (E) Relative SDH activity in isolated mitochondria compared to WT. Mitochondria were isolated from cells grown in SC media plus 2% raffinose / 0.2 % glucose for 24 h (midlog) and 48 h (stationary). Data are shown as mean ± SD is shown (N=3; ** p<0.05). (F) Blue-Native (BN) PAGE analysis to visualize protein complexes. Mitochondria isolated from the strains harvested at late-log phase were solubilized with 1% digitonin. After clarification, soluble fractions were separated on BN-PAGE and then transferred to membranes for immunoblotting. Sdh1, a FAD-containing subunit of SDH; F1β, a subunit of ATP synthase (Complex V, CV) in oxidative phosphorylation. The band highlighted by ** is the Sdh1 assembly intermediate. This band is visualized by antisera to Sdh1 but not Sdh2. See also Figures S1 and S2.
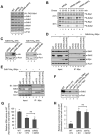
Figure 2. Sdh6 or Sdh7 functions are linked to the Fe/S Sdh2 subunit
(A) FAD-containing Sdh1 was visualized by UV excitation. Other proteins visualized by immunoblotting include Mdh1, malate dehydrogenase and Por1, porin (loading control). (B) 35S-methionine labeled proteins were incubated with isolated mid-log vs. stationary phase mitochondria for 30 min (pulse), followed by blocking protein import with valinomycin for 30 and 60 min (chase), respectively. Radiolabeled proteins were resolved on SDS-PAGE and detected by autoradiography. (C) Sdh6-His6-3HA or Sdh7-His6-3HA under their own endogenous promoters was expressed from plasmids in cells lacking either Sdh2 or Sdh4 along with endogenous Sdh6 or Sdh7 depleted, respectively. Steady-state levels are shown by immunoblotting. (D) Coimmunoprecipitation of Sdh6-His6-2Myc after crosslinking. Mitochondria were solubilized with 1% digitonin in the presence of 1 mM dithiobis[succinimidylpropionate]. The cross-linking reaction was stopped with Tris buffer (pH 7.4), and the supernatants were absorbed to anti-Myc antibody-conjugated magnetic beads. Bound substances to Myc beads were resolved on SDS-PAGE and detected by immunoblotting. Input, 4% of total lysates; Aco1, FeS aconitase. (E) Standard co-immunoprecipitation of Sdh7-His6-2Myc was performed with isolated mitochondria without crosslinking. Input, 2% of total lysates. (F) Steady-state levels of Sdh2 in _sdh1_Δ mutants with overexpression of proteins indicated. Yap1, transcription factor up-regulating oxidative stress response genes. (G) SDH activity in _sdh6_Δ mutants with SDH2 overexpression was detected as described in Figure 1E. Data are represented as mean ± SD (N=3; **p < 0.05; ns, not significant). (H) Succinate levels in _sdh7_Δ mutants with SDH2 overexpression were measured as described in Figure 1D. Mean ± SEM is shown (N=6; **p < 0.05; ns, not significant). See also Figure S3.
Figure 3. Exogenous antioxidants rescue the growth defect of _sdh6_Δ and _sdh7_Δ mutants
(A) Cells harboring either empty vector (EV) or high-copy YAP1 plasmid were spotted on SC media lacking leucine by 10-fold serial dilutions and incubated at 30°C. (B) 10-fold serial dilutions of cells were spotted on SC medium with the indicated carbon sources ± 5 mM N-acetyl cysteine or 2 mM glutathione and incubated at 30°C. (C) Succinate levels in cells overexpressing YAP1 were measured as described in Figure 1D. Cells were harvested at OD600 = 1. Mean ± SEM is shown (N=6; *p<0.005).
Figure 4. _sdh6_Δ and _sdh7_Δ mutants are sensitive to oxidative stress
(A) 10-fold serial dilutions of cells were spotted on SC media ± 2 mM paraquat with indicated carbon sources and incubated at 30°C. (B) Steady-state levels of proteins in mitochondria isolated from strains cultured in the presence of 2 mM paraquat. Yah1, ferredoxin of the mitochondrial matrix. (C) Aconitase activity specific to _cis_-aconitate conversion in isolated mitochondria. Data are shown as mean ± SD (N=3). (D) Pre-cultures grown up to late-log phase in YPD media were diluted 2-fold, followed by addition of 6 mM H2O2 and incubated for 2 h at 30°C. Cells were washed with sterile water and 10-fold serial dilutions were spotteed on YPD plate, followed by incubation at 30°C. (E) Enhanced respiratory growths of _sdh6_Δ mutants and _sdh7_Δ mutants with iron supplementation 10-fold serial dilutions of cells were spotted on SC media ± FeCl2 or ZnCl2 as concentrations indicated, and then incubated at 30°C.
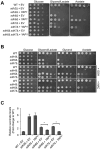
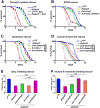
Figure 5. dSdhaf3 mutants are sensitive to oxidative stress and display reduced levels of SdhB, reduced SDH activity, and motility defects
Five-day old w1118 control (solid line) and dSdhaf3 mutant (dotted line) males were transferred to vials with (A) 5% ethanol, 1% agar in PBS, (B) 30 mM paraquat in semi-defined medium, or (C) 100% O2 with standard medium, and living animals were scored daily. Homozygous SdhB12081 mutants (dashed line) were included in the hyperoxia experiment. Each graph was compiled from 3-5 experiments, using a total of 15-21 vials with 20 animals per vial. Error bars represent ±SEM. dSdhaf3 mutants are significantly more sensitive than controls under each condition, p<0.001. (D) GC/MS was used to compare the relative levels of small metabolites in wild-type controls (grey boxes) and dSdhaf3 mutants (white boxes). N=12 samples from two independent experiments with 20 flies/sample (5-day old). ***p<0.001. (E) Proteins were extracted from mitochondria isolated from w1118 controls_, dSdhaf3_ mutants, or UAS-dSdhaf3/+ transformants, and analyzed by immunoblotting to detect SdhA, SdhB, and ATPα, (subunit of complex V). (F) A continuous colorimetric assay was used to measure SDH enzyme activity in extracts of purified mitochondria from w1118 controls, dSdhaf3 mutants, and UASdSdhaf3/+ transformants. ***p<0.001. (G) Proteins from purified mitochondria were extracted from w1118 controls and dSdhaf3 mutants, fractionated by non-denaturing PAGE, and analyzed for SDH and Complex IV activity. (H) Control w1118 flies and dSdhaf3 mutants were tested for motility in three independent experiments using a total of 18 vials with 20 adults/vial at 1, 2, 3, or 4-weeks of age. Climbing ability is reported as the number of flies that climbed above a line drawn 4 cm above the bottom of the vial five seconds after being tapped to the bottom. *p<0.05, ***p<0.001
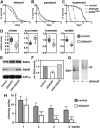
Figure 6. dSdhaf3 function is required in the muscles and nervous system
Five-day old w1118 control (blue solid line) and dSdhaf3 mutant (red dotted line) males were transferred to vials with 100% O2 with standard medium, and living animals were scored daily. (A) 0.1% N-acetyl cysteine was added to the culture medium for a quarter of the vials. (B) Expression of Sod2 using the ubiquitous Act5C-GAL4 driver (Act>Sod2; purple line) partially rescues the hyperoxia sensitivity of dSdhaf3 mutants. Both NAC treatment and Sod2 expression significantly rescue the hyperoxia sensitivity of dSdhaf3 mutants, p<0.001. (C) Expression of wild-type dSdhaf3_ using the ubiquitous Act5C-GAL4 driver (_Act>dSdhaf3; purple line) rescues the hyperoxia sensitivity of dSdhaf3 mutants (p<0.001). (D) The muscle-specific C57-GAL4_ driver provides minor, but significant (p<0.01), rescue of the hyperoxia sensitivity of _dSdhaf3_ mutants (purple line) relative to the control that carries the _UAS-dSdhaf3_ transgene alone (green line), while the CNS-specific _elav-GAL4_ driver provides more efficient rescue (orange line)(p<0.001). Expression of wild-type _dSdhaf3_ by using either the ubiquitous _Act5C-GAL4_ driver (E, purple), the muscle-specific _C57-GAL4_ driver (F, purple), or the CNS-specific _elavGAL4_ driver (F, orange) rescues the climbing defect in _dSdhaf3_ mutants. The _Act>dSdhaf3 rescue in C and E was performed in females, other rescue studies were performed in males (A,B,D,F). The apparent partial rescue of dSdhaf3 mutants by a single copy of the UAS-dSdhaf3 transgene (C,D, green line) appears to be due to genetic background since UAS-dSdhaf3 transformants have normal levels of SdhB and SDH activity (E,F). Each graph was compiled from two experiments with a total of 10 vials with 20 animals per vial. ***p<0.001
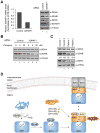
Figure 7. SDHB is destabilized in human cells with reduced levels of SDHAF1
(A) Relative SDHAF1 mRNA levels in HEK293 cells 72 h after SDHAF1 knockdown using siRNA (left panel) and steady-state levels of proteins from total cell lysates (right panel). (B) HEK293 cells were treated with either control siRNA or SDHAF1 siRNA. Paraquat was added to cultures 24 h after siRNA transfection. Total cell lysates were obtained 48 h after paraquat treatment. (C) Steady-state levels of proteins in mitochondria isolated from control fibroblasts and patient fibroblasts harboring mutations on SDHAF1 (Ohlenbusch et al., 2012). The indicated percentages are relative levels of SDHB normalized to ATP5A levels by densitometry. (D) Model of the role of Sdh6(SDHAF1) and Sdh7(SDHAF3) in maturation of Sdh2(SDHB). Sdh6 and Sdh7 associate with Sdh2 within a Sdh1/Sdh2 intermediate. Sdh1 maturation requires covalent flavinylation by Sdh5 followed by formation of the Sdh1/Sdh2 subcomplex that is chaperoned by Sdh8 (see accompanying paper VanVraken et al. 2014).
Similar articles
- The assembly of succinate dehydrogenase: a key enzyme in bioenergetics.
Moosavi B, Berry EA, Zhu XL, Yang WC, Yang GF. Moosavi B, et al. Cell Mol Life Sci. 2019 Oct;76(20):4023-4042. doi: 10.1007/s00018-019-03200-7. Epub 2019 Jun 24. Cell Mol Life Sci. 2019. PMID: 31236625 Free PMC article. Review. - Analysis of SDHAF3 in familial and sporadic pheochromocytoma and paraganglioma.
Dwight T, Na U, Kim E, Zhu Y, Richardson AL, Robinson BG, Tucker KM, Gill AJ, Benn DE, Clifton-Bligh RJ, Winge DR. Dwight T, et al. BMC Cancer. 2017 Jul 24;17(1):497. doi: 10.1186/s12885-017-3486-z. BMC Cancer. 2017. PMID: 28738844 Free PMC article. - SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration.
Van Vranken JG, Bricker DK, Dephoure N, Gygi SP, Cox JE, Thummel CS, Rutter J. Van Vranken JG, et al. Cell Metab. 2014 Aug 5;20(2):241-52. doi: 10.1016/j.cmet.2014.05.012. Epub 2014 Jun 19. Cell Metab. 2014. PMID: 24954416 Free PMC article. - The mitochondrial LYR protein SDHAF1 is required for succinate dehydrogenase activity in Arabidopsis.
Li Y, Belt K, Alqahtani SF, Saha S, Fenske R, Van Aken O, Whelan J, Millar AH, Murcha MW, Huang S. Li Y, et al. Plant J. 2022 Apr;110(2):499-512. doi: 10.1111/tpj.15684. Epub 2022 Feb 26. Plant J. 2022. PMID: 35080330 Free PMC article. - Sequence diversity and conservation in factors influencing succinate dehydrogenase flavinylation.
Huang S, Millar AH. Huang S, et al. Plant Signal Behav. 2013 Feb;8(2):e22815. doi: 10.4161/psb.22815. Epub 2012 Nov 15. Plant Signal Behav. 2013. PMID: 23154507 Free PMC article. Review.
Cited by
- Caffeic Acid Phenethyl Ester Protects Kidney Mitochondria against Ischemia/Reperfusion Induced Injury in an In Vivo Rat Model.
Kamarauskaite J, Baniene R, Trumbeckas D, Strazdauskas A, Trumbeckaite S. Kamarauskaite J, et al. Antioxidants (Basel). 2021 May 8;10(5):747. doi: 10.3390/antiox10050747. Antioxidants (Basel). 2021. PMID: 34066715 Free PMC article. - Genomics of Secondarily Temperate Adaptation in the Only Non-Antarctic Icefish.
Rivera-Colón AG, Rayamajhi N, Minhas BF, Madrigal G, Bilyk KT, Yoon V, Hüne M, Gregory S, Cheng CHC, Catchen JM. Rivera-Colón AG, et al. Mol Biol Evol. 2023 Mar 4;40(3):msad029. doi: 10.1093/molbev/msad029. Mol Biol Evol. 2023. PMID: 36806940 Free PMC article. - ACP Acylation Is an Acetyl-CoA-Dependent Modification Required for Electron Transport Chain Assembly.
Van Vranken JG, Nowinski SM, Clowers KJ, Jeong MY, Ouyang Y, Berg JA, Gygi JP, Gygi SP, Winge DR, Rutter J. Van Vranken JG, et al. Mol Cell. 2018 Aug 16;71(4):567-580.e4. doi: 10.1016/j.molcel.2018.06.039. Mol Cell. 2018. PMID: 30118679 Free PMC article. - The assembly of succinate dehydrogenase: a key enzyme in bioenergetics.
Moosavi B, Berry EA, Zhu XL, Yang WC, Yang GF. Moosavi B, et al. Cell Mol Life Sci. 2019 Oct;76(20):4023-4042. doi: 10.1007/s00018-019-03200-7. Epub 2019 Jun 24. Cell Mol Life Sci. 2019. PMID: 31236625 Free PMC article. Review. - The unassembled flavoprotein subunits of human and bacterial complex II have impaired catalytic activity and generate only minor amounts of ROS.
Maklashina E, Rajagukguk S, Iverson TM, Cecchini G. Maklashina E, et al. J Biol Chem. 2018 May 18;293(20):7754-7765. doi: 10.1074/jbc.RA118.001977. Epub 2018 Apr 2. J Biol Chem. 2018. PMID: 29610278 Free PMC article.
References
- Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochim Biophys Acta. 2011;1807:1432–1443. - PubMed
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers, et al. Mutations in SDHD, a mitochondrial complex II gene in hereditary paraganglioma. Science. 2000;287:848–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01 GM094232/GM/NIGMS NIH HHS/United States
- R01 ES003817/ES/NIEHS NIH HHS/United States
- R01 GM110755/GM/NIGMS NIH HHS/United States
- R01 ES03817/ES/NIEHS NIH HHS/United States
- T32 GM007464/GM/NIGMS NIH HHS/United States
- R01 GM094232/GM/NIGMS NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous