Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer - PubMed (original) (raw)
. 2014 Jul 3;158(1):185-197.
doi: 10.1016/j.cell.2014.06.003. Epub 2014 Jun 19.
Wantong Yao # 1 2, Haoqiang Ying # 1 2, Sujun Hua 1, Alison Liewen 1, Qiuyun Wang 1, Yi Zhong 3, Chang-Jiun Wu 1, Anguraj Sadanandam 4 5, Baoli Hu 1, Qing Chang 2, Gerald C Chu 6, Ramsey Al-Khalil 1, Shan Jiang 1, Hongai Xia 1, Eliot Fletcher-Sananikone 1, Carol Lim 1, Gillian I Horwitz 1, Andrea Viale 1 2, Piergiorgio Pettazzoni 1 2, Nora Sanchez 1 2, Huamin Wang 7, Alexei Protopopov 3, Jianhua Zhang 3, Timothy Heffernan 3, Randy L Johnson 8, Lynda Chin 1 3, Y Alan Wang 1, Giulio Draetta 1 2 3, Ronald A DePinho 9
Affiliations
- PMID: 24954535
- PMCID: PMC4109295
- DOI: 10.1016/j.cell.2014.06.003
Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer
Avnish Kapoor et al. Cell. 2014.
Erratum in
- Yap1 Activation Enables Bypass of Oncogenic Kras Addiction in Pancreatic Cancer.
Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, Chang Q, Chu GC, Al-Khalil R, Jiang S, Xia H, Fletcher-Sananikone E, Lim C, Horwitz GI, Viale A, Pettazzoni P, Sanchez N, Wang H, Protopopov A, Zhang J, Heffernan T, Johnson RL, Chin L, Wang YA, Draetta G, DePinho RA. Kapoor A, et al. Cell. 2019 Nov 14;179(5):1239. doi: 10.1016/j.cell.2019.10.037. Cell. 2019. PMID: 31730860 Free PMC article. No abstract available.
Abstract
Activating mutations in KRAS are among the most frequent events in diverse human carcinomas and are particularly prominent in human pancreatic ductal adenocarcinoma (PDAC). An inducible Kras(G12D)-driven mouse model of PDAC has established a critical role for sustained Kras(G12D) expression in tumor maintenance, providing a model to determine the potential for and the underlying mechanisms of Kras(G12D)-independent PDAC recurrence. Here, we show that some tumors undergo spontaneous relapse and are devoid of Kras(G12D) expression and downstream canonical MAPK signaling and instead acquire amplification and overexpression of the transcriptional coactivator Yap1. Functional studies established the role of Yap1 and the transcriptional factor Tead2 in driving Kras(G12D)-independent tumor maintenance. The Yap1/Tead2 complex acts cooperatively with E2F transcription factors to activate a cell cycle and DNA replication program. Our studies, along with corroborating evidence from human PDAC models, portend a novel mechanism of escape from oncogenic Kras addiction in PDAC.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
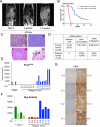
Figure 1. Spontaneous pancreatic tumor relapse after complete regression upon KrasG12D inactivation
(A) Representative MRI scan shows initial tumor regression (3 weeks) but subsequent relapse (14 weeks) after doxy withdrawal. (B) Kaplan-Meier overall survival analysis for iKras; p53L/+ mice after doxy withdrawal. On: mice were fed with doxy. Off: Mice with advanced PDAC were switched to doxy-free water 8-15 weeks after on doxy and observed for relapse. (C) Histopathological characterization of the relapse tumors showing poorly differentiated (i) or sarcomatoid (ii) relapse tumors, with liver (iii) and lung (iv) metastasis (denoted by arrow). (D) Quantitative comparison of histopathological features between primary and relapse tumors. (E) qRT-PCR for KrasG12D transgene shows expression in relapse tumors. Data represented as relative normalized expression. (F) Measurement of Ras activity in relapse tumors. For E and F; Two independent iKras cells were maintained in the presence (+) or absence (-) of doxy for 24 hr and used as controls. Error bars represent SD of duplicate samples. (G) The relapse tumors were stained with antibodies against pErk. See also Figure S1.
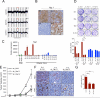
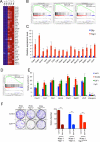
Figure 2. Yap1 is amplified in iKras− relapse tumors and required for tumor growth
(A) aCGH plots of relapse tumors shows that the 9qA1 locus containing Yap1 is focally amplified (denoted by red arrow) in E-1, E-2 and E-5. Normalized log2 ratio for each probe are plotted. (B) IHC for Yap1 in relapse tumors. Note increased Yap1 expression in 9qA1 amplicon+ tumors, E-1, E-2 and E-5 but not 9qA1 amplicon− tumor E-3. (C) qRT-PCR for Yap1 in relapse tumors. Relative expression levels normalized to reference gene. Error bars represent SD of the mean. (D) Representative wells of the clonogenic growth assay upon knockdown of Yap1 by two independent shRNAs primary cultures in Yap1 amplicon+ tumors (E-1 and E-2) and the iKras+ tumors (E-9 and E-10). Non-targeting shRNA (sh_Scr) was used as control. Quantification of cell growth is shown at the bottom. Error bars represent SD of triplicate wells, ***p < 0.001. (E) Xenograft tumor growth of cell cultures derived from E-2 expressing two independent Yap1 shRNA or control shRNA. Tumor volume was measured at the days indicated, data shown is representative of results from 2 independent experiments (n=5 per group). Error bars represent SD of the mean, **p < 0.01; ***p < 0.001. (F) IHC for proliferation marker Ki-67 and Yap1 in E-2 xenograft tumors expressing Yap1 shRNA described in (E) (G) Quantification of IHC staining for Ki-67 displayed as percentage of cells positive for Ki-67 staining. Error bars represent SD of the mean of 5 random fields, ***p<0.001). See also Figure S2.
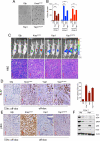
Figure 3. Enforced Yap1 expression enables tumor maintenance upon KrasG12D extinction in PDAC
(A) Representative wells of anchorage independent growth assay demonstrating the ability of Yap1 or Yap1S127A to substitute for oncogenic Kras in promoting cell growth of iKras cells. Growth of Gfp infected cells was impaired in the absence of oncogenic Kras. (B) Quantification of anchorage independent growth assay in three independently derived iKras cells (grown offdoxy, KrasG12D off), For each condition, five random fields were counted. Error bars represent SD of the mean, ***p < 0.001. (C) Yap1 mediated bypass of tumor regression (upon inactivation of KrasG12D) in orthotopic xenografts generated from iKras cells used in (A). Mice (n=10 per group) were kept on-doxy for 7 days post-implantation and then switched to off-doxy. Top: Tumor growth off-doxy was visualized by bioluminescent imaging at 4 weeks off-doxy, except for KrasG12V for which image is taken 2 weeks after switching animals off-doxy. Gfp expressing cells regressed upon KrasG12D inactivation. Bottom: H/E of representative tumors is shown, Scale bar: 100μM. (D&E) IHC for Ki-67 (D, quantified on the right) and pErk (E). Gfp expressing tumor 72 hr after doxy was used as a negative control. Note proficient proliferation (as visualized by Ki-67 staining in D) and lack of MAPK activation (as visualized by low pErk staining in E) in the Yap1 expressing tumors. (F) Signaling status of key RAS effectors in short term cultures derived from three independent Yap1 expressing orthotopic tumors described in 3C. See also Figure S3.
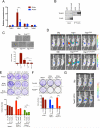
Figure 4. Interaction of Yap1 with Tead2 is critical for it's ability to bypass KrasG12D- dependence
(A) qRT-PCR for expression of Tead family of transcription factors (Tead1-4) in iKras− and iKras+ relapse tumors. Error bars represent SD of the mean, ***p < 0.001. (B) Tead2 interacts with Yap1 in Yap1 (Flag-tagged) expressing cells (described in 3C). Input (25%) is used as a reference. (C) Sustained expression of wild-type Yap1 but not TEAD binding defective Yap1 mutants (YapS94A and Yap1Δ60-89) can promote anchorage independent growth of iKras cells offdoxy. For each condition, five random fields were counted. Error bars represent SD of the mean, ***p < 0.001. (D) Mutation in Tead binding domain of Yap1 (S94A) dramatically decreases the ability of Yap1 or Yap1S127A to substitute for oncogenic Kras in vivo. Representative images shown at 6 weeks off-doxy (n=5 per group). (E) Representative wells (top) of the clonogenic growth assay upon knockdown of Tead2 by two independent shRNAs in Yap1 (or Yap1S127A) expressing cells (described in 3C). Quantification of cell growth is shown below. Error bars represent SD of triplicate wells, ***p < 0.001. (F) Dominant negative Tead2 (Tead2DN) selectively suppresses proliferation of Yap1 (or Yap1S127A) expressing cells but not the KrasG12D expressing iKras cells. Quantification of cell growth is shown below. Error bars represent SD of triplicate wells, ***p < 0.001. (G) Transcriptionally active form of Tead2 (Tead2-VP16) can substitute oncogenic Kras for in vivo tumor growth. Representative images are shown at 6 weeks off-doxy (n=5 per group). See also Figure S4.
Figure 5. Yap1/Tead2 cooperate with E2F to promote a cell cycle and DNA replication
(A) Representative heat maps of the cell cycle and DNA replication genes enriched in Yap1 bypassed tumors compared to control (Gfp, off-doxy for 24 hr). Expression levels shown are representative of log2 values of each replicate. Red signal denotes higher expression relative to the mean expression level within the group and blue signal denotes lower expression relative to the mean expression level within the group. (B) Representative GSEA enrichment plots showing overrepresentation of indicated gene set categories among differentially expressed genes in Yap1 tumors compared with Gfp expressing tumors (24 hr, off-doxy). NES denotes normalized enrichment score. (C) qRT-PCR validation of representative differentially expressed genes in Yap expressing tumors. (D) GSEA enrichment plots showing E2F motif containing gene signatures in the differentially expressed genes in Yap1 expressing tumors compared with control. (E) ChIP showing YAP1 and TEAD2 occupancy at E2F1 bound promoters of several representative genes..No occupancy was seen in the control intergenic region (lacking any putative E2F/TEAD binding sites). IgG served as specificity control for the antibody. Bars represent enrichment at target regions in the promoter relative to the 3’ region of each gene. (F) Dominant negative E2F1 (E2F1DN) suppresses proliferation of Yap1 (or Yap1S127A) expressing cells. Quantification from a representative experiment is shown on the right. Error bars represent SD of triplicate wells, ***p < 0.001. See also Figure S5.
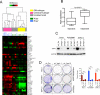
Figure 6. KrasG12D-independent relapse tumors resemble the quasimesenchymal-subtype of human PDAC
(A) Hierarchical clustering of murine PDAC iKras cells and the relapse tumors into different PDAC subtypes using PDAssigner genes (Collisson et al., 2011). Subtype analysis found statistically significant association between iKras− relapse tumors and the QM subtype while the iKras+ tumors are associated with the classical subtype (Chi square test, p-value=0.01; Collisson et al., 2011). The subtype identity of the samples (in gray) is not apparent. (B) Gene expressiondata reanalyzed from Collisson et al., 2011 showing YAP1 expression is significantly higher in the Kras-independent lines compared to Kras-dependent human PDAC cells. The y-axis indicates gene expression data expressed as log2 median centered intensity. Boxed bars indicate the medians. (C) Western blots validating the knockdown of YAP1 in the indicated human PDAC cell lines by two independent shRNAs. (D) Representative wells (top) of clonogenic growth of Kras-independent QM human PDAC cells (Panc1 and PaTu8988T) and the Wild-type KRAS cell line BxPC-3 upon YAP1 knockdown. Quantification (bottom) from a representative experiment is shown on the right. Error bars represent SD of triplicate wells, ***p < 0.001.
Comment in
- YAP1 takes over when oncogenic K-Ras slumbers.
Greten FR. Greten FR. Cell. 2014 Jul 3;158(1):11-2. doi: 10.1016/j.cell.2014.06.021. Cell. 2014. PMID: 24995973
Similar articles
- YAP1 takes over when oncogenic K-Ras slumbers.
Greten FR. Greten FR. Cell. 2014 Jul 3;158(1):11-2. doi: 10.1016/j.cell.2014.06.021. Cell. 2014. PMID: 24995973 - KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma.
Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, Liu J, Lemischka IR, Hung MC, Chiao PJ. Ling J, et al. Cancer Cell. 2012 Jan 17;21(1):105-20. doi: 10.1016/j.ccr.2011.12.006. Cancer Cell. 2012. PMID: 22264792 Free PMC article. - YAP1 and TAZ Control Pancreatic Cancer Initiation in Mice by Direct Up-regulation of JAK-STAT3 Signaling.
Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. Gruber R, et al. Gastroenterology. 2016 Sep;151(3):526-39. doi: 10.1053/j.gastro.2016.05.006. Epub 2016 May 20. Gastroenterology. 2016. PMID: 27215660 Free PMC article. - Central role of Yes-associated protein and WW-domain-containing transcriptional co-activator with PDZ-binding motif in pancreatic cancer development.
Rozengurt E, Eibl G. Rozengurt E, et al. World J Gastroenterol. 2019 Apr 21;25(15):1797-1816. doi: 10.3748/wjg.v25.i15.1797. World J Gastroenterol. 2019. PMID: 31057295 Free PMC article. Review. - KRAS, YAP, and obesity in pancreatic cancer: A signaling network with multiple loops.
Eibl G, Rozengurt E. Eibl G, et al. Semin Cancer Biol. 2019 Feb;54:50-62. doi: 10.1016/j.semcancer.2017.10.007. Epub 2017 Oct 24. Semin Cancer Biol. 2019. PMID: 29079305 Free PMC article. Review.
Cited by
- Beyond the Genomic Mutation: Rethinking the Molecular Biomarkers of K-RAS Dependency in Pancreatic Cancers.
Mottini C, Cardone L. Mottini C, et al. Int J Mol Sci. 2020 Jul 16;21(14):5023. doi: 10.3390/ijms21145023. Int J Mol Sci. 2020. PMID: 32708716 Free PMC article. Review. - Knockdown of SOX18 inhibits the proliferation, migration and invasion of hepatocellular carcinoma cells.
Wang G, Wei Z, Jia H, Zhao W, Yang G, Zhao H. Wang G, et al. Oncol Rep. 2015 Sep;34(3):1121-8. doi: 10.3892/or.2015.4112. Epub 2015 Jul 7. Oncol Rep. 2015. PMID: 26151573 Free PMC article. - YAP1 oncogene is a context-specific driver for pancreatic ductal adenocarcinoma.
Tu B, Yao J, Ferri-Borgogno S, Zhao J, Chen S, Wang Q, Yan L, Zhou X, Zhu C, Bang S, Chang Q, Bristow CA, Kang Y, Zheng H, Wang H, Fleming JB, Kim M, Heffernan TP, Draetta GF, Pan D, Maitra A, Yao W, Gupta S, Ying H. Tu B, et al. JCI Insight. 2019 Nov 1;4(21):e130811. doi: 10.1172/jci.insight.130811. JCI Insight. 2019. PMID: 31557131 Free PMC article. - Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine.
Moya IM, Halder G. Moya IM, et al. Nat Rev Mol Cell Biol. 2019 Apr;20(4):211-226. doi: 10.1038/s41580-018-0086-y. Nat Rev Mol Cell Biol. 2019. PMID: 30546055 Review. - RAS degraders: The new frontier for RAS-driven cancers.
Escher TE, Satchell KJF. Escher TE, et al. Mol Ther. 2023 Jul 5;31(7):1904-1919. doi: 10.1016/j.ymthe.2023.03.017. Epub 2023 Mar 21. Mol Ther. 2023. PMID: 36945775 Free PMC article. Review.
References
- Adams PD, Kaelin WG., Jr. The cellular effects of E2F overexpression. Current topics in microbiology and immunology. 1996;208:79–93. - PubMed
- Berns K, Bernards R. Understanding resistance to targeted cancer drugs through loss of function genetic screens. Drug resistance updates : reviews and commentaries in Antimicrobial and Anticancer chemotherapy. 2012;15:268–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA16672/CA/NCI NIH HHS/United States
- R56 DK094865/DK/NIDDK NIH HHS/United States
- R01 DK102611/DK/NIDDK NIH HHS/United States
- P01CA117969/CA/NCI NIH HHS/United States
- 5U01CA084313/CA/NCI NIH HHS/United States
- R56DK094865/DK/NIDDK NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U01 CA084313/CA/NCI NIH HHS/United States
- DH_/Department of Health/United Kingdom
- P01 CA117969/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous