Pre-eclampsia part 1: current understanding of its pathophysiology - PubMed (original) (raw)
Review
Pre-eclampsia part 1: current understanding of its pathophysiology
Tinnakorn Chaiworapongsa et al. Nat Rev Nephrol. 2014 Aug.
Abstract
Pre-eclampsia is characterized by new-onset hypertension and proteinuria at ≥20 weeks of gestation. In the absence of proteinuria, hypertension together with evidence of systemic disease (such as thrombocytopenia or elevated levels of liver transaminases) is required for diagnosis. This multisystemic disorder targets several organs, including the kidneys, liver and brain, and is a leading cause of maternal and perinatal morbidity and mortality. Glomeruloendotheliosis is considered to be a characteristic lesion of pre-eclampsia, but can also occur in healthy pregnant women. The placenta has an essential role in development of this disorder. Pathogenetic mechanisms implicated in pre-eclampsia include defective deep placentation, oxidative and endoplasmic reticulum stress, autoantibodies to type-1 angiotensin II receptor, platelet and thrombin activation, intravascular inflammation, endothelial dysfunction and the presence of an antiangiogenic state, among which an imbalance of angiogenesis has emerged as one of the most important factors. However, this imbalance is not specific to pre-eclampsia, as it also occurs in intrauterine growth restriction, fetal death, spontaneous preterm labour and maternal floor infarction (massive perivillous fibrin deposition). The severity and timing of the angiogenic imbalance, together with maternal susceptibility, might determine the clinical presentation of pre-eclampsia. This Review discusses the diagnosis, classification, clinical manifestations and putative pathogenetic mechanisms of pre-eclampsia.
Conflict of interest statement
Disclosure: The authors report no conflicts of interest.
Figures
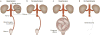
Figure 1. An experiment supporting the concept that hypertension in pregnancy represents a uteroplacental response to ischaemia
A. In the Goldblatt model of renovascular hypertension, clamping the renal artery leads to development of hypertension through renal ischaemia in nonpregnant animals. B. By contrast, clamping the aorta below the renal arteries does not induce hypertension in nonpregnant animals. C. Aortic clamping in pregnant animals leads to hypertension. D. After hysterectomy, however, hypertension can no longer be elicited by aortic clamping, suggesting that the ischaemic pregnant uterus is the source of signals that lead to maternal systemic hypertension. Permission obtained from Semin. Perinatol. 12, Romero, R. et al. Toxemia: new concepts in an old disease, 302–323 © Elsevier (1988).
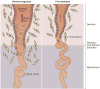
Figure 2. Failure of physiological transformation of the spiral arteries is implicated in pre-eclampsia
A. In a normal pregnancy, physiological transformation of the myometrial segment of the spiral artery occurs. Trophoblast cells extend to both the decidual segment and one-third of the myometrial segment of the spiral artery. Both the arterial media and endothelium are destroyed by trophoblasts, converting the arteries into wide-calibre vessels and increasing the delivery of blood to the intervillous space. B. In pregnancies affected by pre-eclampsia, a key feature associated with the failure of physiological transformation of the spiral arteries is lack of invasion of the trophoblasts into the myometrial segment of the spiral artery. The resulting lack of transformation of blood vessels results in narrow spiral arteries, a disturbed pattern of blood flow and reduced uteroplacental perfusion. Permission obtained from Nature Publishing Group © Moffett-King, A. et al. Nat. Rev. Immunol. 2, 656–663 (2002).
Figure 3. Transformed and nontransformed spiral arteries in the myometrium
A. Transformed spiral arteries are characterized by the presence of intramural trophoblasts (arrowheads) and fibrinoid degeneration (arrows) of the arterial wall. B. Nontransformed spiral arteries lack intramural trophoblasts and fibrinoid degeneration, and retain intact arterial contours. Arrowheads indicate the presence of trophoblasts in myometrium, but not in the wall of the spiral artery. Both images stained with cytokeratin 7 (brown) and periodic acid–Schiff (pink), magnification ×200. Permission obtained from the NIH © Espinoza, J. et al. J. Perinat. Med. 34, 447–458 (2006).
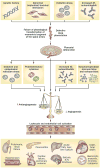
Figure 4. Integrated model of the complex pathophysiology of pre-eclampsia
Genetic (including maternal–fetal genotype incompatibility) and environmental (preconception exposure to paternal antigens) factors disrupt pregnancy-induced immunomodulation, leading to trophoblast and decidual pathology, shallow endometrial invasion and failure of physiological transformation of the spiral arteries (a disorder of deep placentation). The degree of uterine ischaemia is determined by the severity of the placentation defect and fetal demand on the blood supply. Obstetric disorders occur when these two factors are mismatched. The timing and extent of the mismatch determines the clinical presentation (fetal death, pre-eclampsia with IUGR, IUGR alone and late pre-eclampsia). Pre-eclampsia occurs as a result of adaptive responses involving the release of inflammatory cytokines, anti-AT1 autoantibodies, angiogenic and antiangiogenic factors and syncytiotrophoblast-derived particles into the maternal circulation. Collectively, these factors induce leukocyte activation, intravascular inflammation, endothelial cell dysfunction and excessive thrombin generation. The multiorgan features of pre-eclampsia result from the consequences of these processes in different target organs. Abbreviations: AT1, type-1 angiotensin II receptor; ER, endoplasmic reticulum; ICH, intracerebral haemorrhage; IUGR, intrauterine growth restriction; PlGF, placental growth factor; ROS, reactive oxygen species; s, soluble; VEGF, vascular endothelial growth factor; VEGFR-1, vascular endothelial growth factor receptor 1.
Similar articles
- Impact of new definitions of preeclampsia at term on identification of adverse maternal and perinatal outcomes.
Lai J, Syngelaki A, Nicolaides KH, von Dadelszen P, Magee LA. Lai J, et al. Am J Obstet Gynecol. 2021 May;224(5):518.e1-518.e11. doi: 10.1016/j.ajog.2020.11.004. Epub 2020 Nov 6. Am J Obstet Gynecol. 2021. PMID: 33166504 - Preeclampsia and eclampsia: the conceptual evolution of a syndrome.
Erez O, Romero R, Jung E, Chaemsaithong P, Bosco M, Suksai M, Gallo DM, Gotsch F. Erez O, et al. Am J Obstet Gynecol. 2022 Feb;226(2S):S786-S803. doi: 10.1016/j.ajog.2021.12.001. Am J Obstet Gynecol. 2022. PMID: 35177220 Free PMC article. Review. - [Pre-eclampsia screening in first and second trimester].
Kang A, Struben H. Kang A, et al. Ther Umsch. 2008 Nov;65(11):663-6. doi: 10.1024/0040-5930.65.11.663. Ther Umsch. 2008. PMID: 18979429 Review. German. - Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial.
Duhig KE, Myers J, Seed PT, Sparkes J, Lowe J, Hunter RM, Shennan AH, Chappell LC; PARROT trial group. Duhig KE, et al. Lancet. 2019 May 4;393(10183):1807-1818. doi: 10.1016/S0140-6736(18)33212-4. Epub 2019 Apr 1. Lancet. 2019. PMID: 30948284 Free PMC article. Clinical Trial. - Pre-eclampsia: its pathogenesis and pathophysiolgy.
Gathiram P, Moodley J. Gathiram P, et al. Cardiovasc J Afr. 2016 Mar-Apr;27(2):71-8. doi: 10.5830/CVJA-2016-009. Cardiovasc J Afr. 2016. PMID: 27213853 Free PMC article. Review.
Cited by
- Potential Use of Anti-Cancer Drugs for Treatment of Preeclampsia by Targeting the miRNA-IGF1R-PI3K-AKT Axis.
Li J, Hou L, Zhao R, Zou L. Li J, et al. Evid Based Complement Alternat Med. 2022 Aug 22;2022:3883082. doi: 10.1155/2022/3883082. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36045666 Free PMC article. Retracted. - A tale of two cell-fates: role of the Hippo signaling pathway and transcription factors in early lineage formation in mouse preimplantation embryos.
Karasek C, Ashry M, Driscoll CS, Knott JG. Karasek C, et al. Mol Hum Reprod. 2020 Sep 1;26(9):653-664. doi: 10.1093/molehr/gaaa052. Mol Hum Reprod. 2020. PMID: 32647873 Free PMC article. Review. - Distinct placental molecular processes associated with early-onset and late-onset preeclampsia.
Ren Z, Gao Y, Gao Y, Liang G, Chen Q, Jiang S, Yang X, Fan C, Wang H, Wang J, Shi YW, Xiao C, Zhong M, Yang X. Ren Z, et al. Theranostics. 2021 Mar 5;11(10):5028-5044. doi: 10.7150/thno.56141. eCollection 2021. Theranostics. 2021. PMID: 33754042 Free PMC article. - The Role of Inflammation in the Pathogenesis of Preeclampsia.
Michalczyk M, Celewicz A, Celewicz M, Woźniakowska-Gondek P, Rzepka R. Michalczyk M, et al. Mediators Inflamm. 2020 Oct 5;2020:3864941. doi: 10.1155/2020/3864941. eCollection 2020. Mediators Inflamm. 2020. PMID: 33082708 Free PMC article. Review. - MiR-519d-3p suppresses invasion and migration of trophoblast cells via targeting MMP-2.
Ding J, Huang F, Wu G, Han T, Xu F, Weng D, Wu C, Zhang X, Yao Y, Zhu X. Ding J, et al. PLoS One. 2015 Mar 24;10(3):e0120321. doi: 10.1371/journal.pone.0120321. eCollection 2015. PLoS One. 2015. PMID: 25803859 Free PMC article.
References
- Lindheimer MD, Roberts JM, Cunningham GC, Chesley L. In: Chesley’s Hypertensive Disorders in Pregnancy. Lindheimer MD, Roberts JM, Cunningham GC, editors. Elsevier; 2009. pp. 1–24.
- Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin. Perinatol. 1988;12:302–323. - PubMed
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. - PubMed
- Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. - PubMed
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical