Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis - PubMed (original) (raw)
Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis
Peter Thomas et al. Endocrinology. 2014 Nov.
Abstract
Recently, we discovered a cDNA in teleost ovarian follicle cells belonging to the zinc transporter ZIP9 subfamily (SLC39A9) encoding a protein with characteristics of a membrane androgen receptor (mAR). Here, we demonstrate that human ZIP9 expressed in MDA-MB-468 breast cancer cells and stably overexpressed in human prostate cancer PC-3 cells (PC-3-ZIP9) also displays the ligand binding and signaling characteristics of a specific, high-affinity mAR. Testosterone treatment of MDA-MB-468 and PC-3-ZIP9 cells caused activation of G proteins and second messenger pathways as well as increases in intracellular free zinc concentrations that were accompanied by induction of apoptosis. [1,2,6,7-(3)H]-testosterone binding and these responses were abrogated in MDA-MB-468 cells after ZIP9 small interfering RNA (siRNA) treatment and absent in PC-3 cells transfected with empty vector, confirming that ZIP9 functions as an mAR. Testosterone treatment caused up-regulation of proapoptotic genes Bax (Bcl-2-associated X protein), p53 (tumor protein p53), and JNK (c-Jun N-terminal kinases) in both cell lines and increased expression of Bax, Caspase 3, and cytochrome C proteins. Treatment with a zinc chelator or a MAPK inhibitor blocked testosterone-induced increases in Bax, p53, and JNK mRNA expression. The results suggest that both androgen signaling and zinc transporter functions of ZIP9 mediate testosterone promotion of apoptosis. ZIP9 is widely expressed in human tissues and up-regulated in malignant breast and prostate tissues, suggesting that it is a potential therapeutic target for treating breast and prostate cancers. These results provide the first evidence for a mechanism mediated by a single protein through which steroid and zinc signaling pathways interact to regulate physiological functions in mammalian cells.
Figures
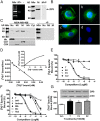
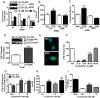
Figure 1.
Membrane androgen binding and expression of ZIP9 in nAR-negative MDA-MB-468 human breast cancer cells. A–C, Expression of ZIP9 mRNA (left) and ZIP9 protein on plasma membranes (6 μg/lane) (right) (A) and ZIP9 protein localization in cells by immunocytochemical analysis (B, a–c; peptide block, d) and in subcellular fractions by Western blot analysis (10 μg/lane) (C). Mkr, 100-bp ladder or molecular weight marker; +P, peptide block; actin, actin loading control; Nu, nuclear fraction; Mi, mitochondrial faction; M, plasma membrane fraction; Ms, microsomal fraction; Cyt, cytoplasmic fraction; His, histone nuclear marker; Cad, cadherin plasma membrane marker; COX, cyclooxygenase mitochondrial marker. The purity of subcellular fractions was confirmed using antibodies to these specific marker proteins. D, Representative saturation analysis and Scatchard plot of specific [3H]-T binding to plasma membranes. E and F, Representative competition curves of steroid binding to plasma membranes expressed as a percentage of maximum [3H]-T binding. T, testosterone; DHT, 5α-dihydrotestosterone; Androst, androstenedione; Cor, cortisol; E2, estradiol-17β; P4, progesterone; mib, mibolerone; Bic, bicalutamide; Hyd, hydroxyflutamide. G, Effects of overnight treatment of MDA-MB-468 cells with 100nM T, P4, and E2 on plasma membrane expression of ZIP9 protein (top) and specific [3H]-T plasma membrane binding (bottom). *, P < .05 compared with vehicle (Veh) control, n = 6.
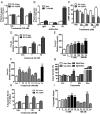
Figure 2.
Effects of androgens on signal transduction pathways and apoptosis through ZIP9 in MDA-MB-468 cells. A, Effects of androgens (100nM) on [35S]-GTPγS binding to plasma membranes. T, testosterone; M, mibolerone. ***, P < .001 compared with vehicle (Veh) control, n = 6. B, Immunoprecipitation of [35S]-GTPγS bound to G protein α-subunits on plasma membranes activated by 100nM T treatment with specific G protein α-subunit antibodies or control IgG. Gs, Gαs antibody; Gi, Gαi antibody. ***, P < .001 compared with corresponding IgG control, n = 6. C, Representative Western blot showing the effects of 15 minutes treatments with T (20, 100nM) and M (100nM) on activation of ERK1/2. EGF, epidermal growth factor-positive control; pERK, phosphorylated ERK. D and E, Effects of T and other steroid treatments on relative intracellular free zinc levels. Py, pyrithione-positive control; TB, charcoal-stripped T bovine serum albumen conjugate; DT, dihydrotestosterone; E2, estradiol-17β; P4, progesterone; Cor, cortisol. ***, P < .001; **, P < .01 compared with Veh control. F and G, Effects of T and other steroids on % apoptotic nuclei in Hoechst (F) and TUNEL (G) assays. H, Effects of treatment with 20nM T and M for 3 and 5 hours on Caspase 3 activity. Stau, staurosporin (600nM); *, P < .05 compared with respective Veh control. I–K, Effects of knockdown of ZIP9 expression by transfection with siRNA (ZIP9) and nontarget siRNA controls (NT) on ZIP9 mRNA expression (I, top, left) and ZIP9 protein expression in plasma membranes (I, top, right) and [3H]-T binding in a single-point assay (bottom) (I) as well as intracellular zinc concentrations (J) and apoptosis (K) in response to 100nM T. Actin, loading controls; Ut, untransfected controls; TB, total binding; NSB, nonspecific binding; SB, specific binding. ***, P < .001; *, P < .05 compared with respective NT-specific binding (G) or Veh NT controls (H), n = 6.
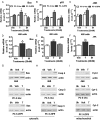
Figure 3.
Membrane androgen binding and expression of ZIP9 in nAR-negative PC-3 human prostate cancer cells overexpressing ZIP9 (PC-3-ZIP9) and in cells transfected with vector alone (PC-3-Vec). A–C, Expression of ZIP9 mRNA (top) and protein on plasma membrane (bottom) (A), by immunocytochemical analysis (B), and ZIP9 protein expression in subcellular fractions by Western blot analysis (C). Protein loading (20 μg/lane) for the Western blotting in C was double that in A to detect minor immunoreactive bands. Mkr, molecular weight marker; M+E, deglycosylated plasma membranes; +P, peptide block; actin, loading control; (see Figure 1 for key to subcellular abbreviations and markers). D, Representative single-point binding assay of specific [3H]-T binding to plasma membranes of PC-3-ZIP9 and PC-3-Vec cells. ***, P < .001 compared with PC-3-Vec cell membranes, n = 6. E, Representative saturation analysis and Scatchard plot of specific [3H]-T binding to cell membranes of PC-3-ZIP9 cells. F and G, Representative competition curves of steroid binding to cell membranes of PC-3-ZIP9 cells expressed as a percentage of maximum [3H]-T binding (see Figure 1 for key to steroid abbreviations).
Figure 4.
Effects androgens on signal transduction pathways and apoptosis in PC-3 human prostate cancer cells overexpressing ZIP9 (PC-3-ZIP9) and in cells transfected with vector alone (PC-3-Vec). A, Effects of treatments with (100nM) testosterone (T) and mibolerone (M) on [35S]-GTPγS binding to plasma membranes. ***, P < .001 compared with vehicle (Veh) control, n = 6. B, Immunoprecipitation of [35S]-GTPγS bound to G protein α-subunits proteins on plasma membranes activated by 100nM T treatment with specific Gα-subunit antibodies or control IgG. Gs, Gαs antibody, Gi, Gαi antibody. ***, P < .001 compared with corresponding IgG control, n = 6. C, Effects of 20 minutes of treatments with T and dihydrotestosterone (DT) (20nM, 100nM) on cAMP production. **, P < .01; *, P < .05 compared with corresponding PC-3-Vec control. D–F, Effects of T and other steroid treatments on relative intracellular free zinc levels. D, Effects of 20nM T treatment on zinc levels in PC-3-ZIP9 and PC-3-Vec cells. ***, P < .001; **, P < .01 compared with corresponding PC-3-Vec controls. E, Concentration-dependent effects of T and effects of T-BSA on zinc levels in PC-3-ZIP9 cells. ***, P < .001; **, P < .01 compared with Veh controls. F, Effects of different steroid treatments (100nM) on intracellular zinc concentrations in PC-3-ZIP9 cells. Py, pyrithione-positive control; TB, T conjugated to bovine serum albumen. ***, P < .001; **, P < .01; *, P < .05 compared with Veh control. G, Effects of treatment with 20nM T and M for 3 and 5 hours on of Caspase 3 activity. Stau, staurosporin. *, P < .05 compared with respective Veh control H and I. Effects of T and other steroids on % apoptotic nuclei by TUNEL (H) and Hoechst (I), in PC-3-ZIP9 cells assays. ***, P < .001; **, P < .01; *, P < .05 compared with respective Veh controls, n = 6 (see Figure 1 for key to steroid abbreviations).
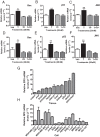
Figure 5.
Membrane testosterone (T) binding and effects of androgens on signal transduction pathways and apoptosis in AR-positive human LNCaP prostate cancer cells (A–C) and in triple-negative MDA-MB-231 breast cancer cells (D–I) overexpressing ZIP9 (231-ZIP9) or vector alone (231-Vec). A–C, Effects of transfection of LNCaP cells with ZIP9 siRNA (ZIP9) or nontarget (NT) siRNA on ZIP9 mRNA (A, top, left) and protein (A, top, right) expression, and specific [3H]-T binding in a single-point binding assay. Ut, untransfected cells; actin, actin loading control. TB, total binding; NSB, nonspecific binding; SB, specific binding. ***, P < .001; **, P < .01 compared with their respective NT controls, n = 6 (A, bottom); on relative intracellular free zinc concentrations, *, P < .05 compared with NT vehicle (Veh) control (B); and on % apoptotic nuclei, ***, P < .001 compared with NT Veh control (C). D, Expression of ZIP9 mRNA (top, left) and protein on plasma membrane (top, right) and specific [3H]-T binding in a single-point binding assay (bottom) in 231-ZIP9 and in 231-vec cells. ***, P < .001 compared with 231-vec control. E, Immunocytochemical analysis of ZIP9 protein expression in 231-vec (Vec) and 231-ZIP9 (ZIP9) cells. F, Two-point competitive binding assay of T and mibolerone (M) (100nM and 1μM, respectively) binding to 231-ZIP9 cell membranes expressed as a percentage of maximum specific [3H]-T-binding. ***, P < .001 compared with Veh control. G–I, Effects of T and M (100nM) on [35S]GTPγS binding to plasma membranes (G), on intracellular zinc concentrations in 231-ZIP9 cells (H), and on % apoptotic nuclei in 231-ZIP9 cells (I). Py, pyrithione-positive zinc control; Stau, staurosporine-positive apoptosis control. ***, P < .001; **, P < .01; *, P < .05 compared with respective Veh controls, n = 6.
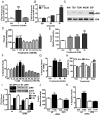
Figure 6.
Effects of testosterone (T) and mibolerone treatments on expression of members of apoptotic pathways in MDA-MB-468 (468), PC-3-ZIP9 and PC-3-Vec cells. A–F, Effects of 24-hour treatments with 20nM T and mibolerone on relative mRNA levels of Bax, p53, and JNK in PC-3-ZIP9 and PC-3-Vec (A–C) and in MDA-MB-468 (D–F) cells measured by q-PCR. **, P < .01 compared with vehicle (Veh) controls; *, P < .05 compared with corresponding PC-3-Vec treatments or Veh control, n = 6. G–I, Effects of 48 hours of treatment with 20nM T or Veh, or 0 hours of no treatment (0 h) on cytosolic expression of Bax and Caspase 3 (Casp3) proteins, and mitochondrial expression of cytochrome C (Cyt-C) protein in MDA-MB-468, PC-3-ZIP9, and PC-3-Vec cells determined by Western blot analysis. Actin, actin loading control.
Figure 7.
Effects of cotreatment with an intracellular zinc chelator (TPEN) or a MAPK inhibitor (PD98059) on testosterone (T) (20nM) up-regulation of Bax, p53, and JNK mRNA expression in MDA-MB-468 (A–C) and PC-3-ZIP9 (D–F) cells after 24 hours of treatment. Veh, vehicle; TP, TPEN; PD, PD98059. **, P < .01; *, P < .05 compared with respective Veh controls. G, Relative expression of ZIP9 mRNA in normal adult human RNA samples. Pitui, pituitary; n = 3. H, Relative ZIP9 mRNA expression in cancer and noncancer cells. Cancer cell lines: MDA-MB-231, MDA-MB-468, SKBR3, T47D, and MCF-7 (breast); LNCaP, PC-3, and DU-145 (prostate); SKOV (ovarian); IMR-32 (neuroblastoma, brain); and Jurkat (T-cell leukemia). Noncancer cells: SIGC (spontaneously immortalized granulosa cells; using rat ZIP9 primers); VSMC (primary vascular smooth muscle cells from human umbilical vein); HUVEC (primary endothelial cells from human umbilical vein); T-cell (human T lymphocytes); and HEK293.
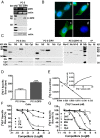
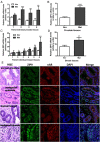
Figure 8.
Relative expression of ZIP9 mRNA in paired normal (Nor) and malignant (Mal) human prostate (A and B) and breast (C and D) tissue biopsies measured by q-PCR. ***, P < .001; **, P < .01; *, P < .05 compared with respective paired normal control (A and C) or respective mean normal controls (B and D). All the malignant prostate biopsy samples were classified as acinar adenocarcinoma (Gleason score 6–7). All the malignant breast biopsy specimens were classified as invasive ductal carcinoma (Nottingham grade 3 or Bloom-Richardson 2–3) (see Supplemental Table 2 for additional details of the malignant biopsy samples). E, Hematoxylin and eosin (H&E) staining and immunohistochemistry of representative normal and malignant prostate (biopsy specimen 1) and breast cancer (biopsy specimen 3) tissues showing localization of ZIP9 and nAR proteins (see Supplemental Figure 2 for additional immunohistochemistry images of these specimens). Image amplification, ×100.
Comment in
- Unzipping androgen action through ZIP9: a novel membrane androgen receptor.
Pascal LE, Wang Z. Pascal LE, et al. Endocrinology. 2014 Nov;155(11):4120-3. doi: 10.1210/en.2014-1749. Endocrinology. 2014. PMID: 25325426 No abstract available.
Similar articles
- (-)-Epicatechin acts as a potent agonist of the membrane androgen receptor, ZIP9 (SLC39A9), to promote apoptosis of breast and prostate cancer cells.
Thomas P, Dong J. Thomas P, et al. J Steroid Biochem Mol Biol. 2021 Jul;211:105906. doi: 10.1016/j.jsbmb.2021.105906. Epub 2021 May 11. J Steroid Biochem Mol Biol. 2021. PMID: 33989703 - ZIP9, a novel membrane androgen receptor and zinc transporter protein.
Thomas P, Converse A, Berg HA. Thomas P, et al. Gen Comp Endocrinol. 2018 Feb 1;257:130-136. doi: 10.1016/j.ygcen.2017.04.016. Epub 2017 May 4. Gen Comp Endocrinol. 2018. PMID: 28479083 Review. - Membrane Androgen Receptors Unrelated to Nuclear Steroid Receptors.
Thomas P. Thomas P. Endocrinology. 2019 Apr 1;160(4):772-781. doi: 10.1210/en.2018-00987. Endocrinology. 2019. PMID: 30753403 Review.
Cited by
- ARe we there yet? Understanding androgen receptor signaling in breast cancer.
Michmerhuizen AR, Spratt DE, Pierce LJ, Speers CW. Michmerhuizen AR, et al. NPJ Breast Cancer. 2020 Sep 25;6:47. doi: 10.1038/s41523-020-00190-9. eCollection 2020. NPJ Breast Cancer. 2020. PMID: 33062889 Free PMC article. Review. - Dietary Phytochemicals in Zinc Homeostasis: A Strategy for Prostate Cancer Management.
Singh CK, Chhabra G, Patel A, Chang H, Ahmad N. Singh CK, et al. Nutrients. 2021 May 30;13(6):1867. doi: 10.3390/nu13061867. Nutrients. 2021. PMID: 34070833 Free PMC article. Review. - ZnT8 Haploinsufficiency Impacts MIN6 Cell Zinc Content and β-Cell Phenotype via ZIP-ZnT8 Coregulation.
Lawson R, Maret W, Hogstrand C. Lawson R, et al. Int J Mol Sci. 2019 Nov 4;20(21):5485. doi: 10.3390/ijms20215485. Int J Mol Sci. 2019. PMID: 31690008 Free PMC article. - Procaspase-3 Overexpression in Cancer: A Paradoxical Observation with Therapeutic Potential.
Boudreau MW, Peh J, Hergenrother PJ. Boudreau MW, et al. ACS Chem Biol. 2019 Nov 15;14(11):2335-2348. doi: 10.1021/acschembio.9b00338. Epub 2019 Jul 16. ACS Chem Biol. 2019. PMID: 31260254 Free PMC article. Review. - Zinc and its binding proteins: essential roles and therapeutic potential.
Kiouri DP, Chasapis CT, Mavromoustakos T, Spiliopoulou CA, Stefanidou ME. Kiouri DP, et al. Arch Toxicol. 2024 Nov 7. doi: 10.1007/s00204-024-03891-3. Online ahead of print. Arch Toxicol. 2024. PMID: 39508885 Review.
References
- Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr Rev. 1998;19:3–17. - PubMed
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. - PubMed
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. Rβ has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous