Transition from metabolic adaptation to maladaptation of the heart in obesity: role of apelin - PubMed (original) (raw)
Transition from metabolic adaptation to maladaptation of the heart in obesity: role of apelin
C Alfarano et al. Int J Obes (Lond). 2015 Feb.
Abstract
Background/objectives: Impaired energy metabolism is the defining characteristic of obesity-related heart failure. The adipocyte-derived peptide apelin has a role in the regulation of cardiovascular and metabolic homeostasis and may contribute to the link between obesity, energy metabolism and cardiac function. Here we investigate the role of apelin in the transition from metabolic adaptation to maladaptation of the heart in obese state.
Methods: Adult male C57BL/6J, apelin knock-out (KO) or wild-type mice were fed a high-fat diet (HFD) for 18 weeks. To induce heart failure, mice were subjected to pressure overload after 18 weeks of HFD. Long-term effects of apelin on fatty acid (FA) oxidation, glucose metabolism, cardiac function and mitochondrial changes were evaluated in HFD-fed mice after 4 weeks of pressure overload. Cardiomyocytes from HFD-fed mice were isolated for analysis of metabolic responses.
Results: In HFD-fed mice, pressure overload-induced transition from hypertrophy to heart failure is associated with reduced FA utilization (P<0.05), accelerated glucose oxidation (P<0.05) and mitochondrial damage. Treatment of HFD-fed mice with apelin for 4 weeks prevented pressure overload-induced decline in FA metabolism (P<0.05) and mitochondrial defects. Furthermore, apelin treatment lowered fasting plasma glucose (P<0.01), improved glucose tolerance (P<0.05) and preserved cardiac function (P<0.05) in HFD-fed mice subjected to pressure overload. In apelin KO HFD-fed mice, spontaneous cardiac dysfunction is associated with reduced FA oxidation (P<0.001) and increased glucose oxidation (P<0.05). In isolated cardiomyocytes, apelin stimulated FA oxidation in a dose-dependent manner and this effect was prevented by small interfering RNA sirtuin 3 knockdown.
Conclusions: These data suggest that obesity-related decline in cardiac function is associated with defective myocardial energy metabolism and mitochondrial abnormalities. Furthermore, our work points for therapeutic potential of apelin to prevent myocardial metabolic abnormalities in heart failure paired with obesity.
Figures
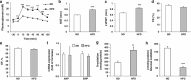
Figure 1
Cardiometabolic profile of HFD-induced obese mice. (a) Glucose tolerance test (GTT) in mice after 18 weeks of exposure to ND (_n_=8) or HFD (_n_=10). (b) Interventricular septum thickness (IVST), (c) LV posterior wall thickness (LVPWT) and (d) fractional shortening (FS) and (e) ejection fraction (EF) were analyzed by two-dimensional guided M-mode echocardiography in ND-fed and HFD-fed mice. (f) Real-time reverse transcriptase (RT)-PCR analysis of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) expression levels in heart tissue from ND-fed (_n_=6) and HFD-fed (_n_=7) mice. (g) Myocardial FA oxidation and (h) glucose oxidation in ND-fed (_n_=7) and HFD-fed (_n_=8) mice. Results are means±s.e.m. **P<0.01; ***P<0.001 vs ND.
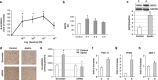
Figure 2
Cardiometabolic reprogramming in obesity-related heart failure: effects of apelin. (a) Representative two-dimensional and M-Mode echocardiographic images of mice subjected to 4 weeks of AB (n_=7) or sham operation (Sham, n_=8) and received 0.1 μmol kg–1 day–1 intraperitoneal apelin or vehicle for 4 weeks. (b) Echocardiographic measurements of interventricular septum thickness in diastole (IVSTd), posterior wall thickness in diastole (PWTd), LV internal diameter in diastole (LVIDd), fractional shortening (FS) and ejection fraction (EF) in sham or AB HFD-fed mice treated with vehicle or apelin. (c) Real-time reverse transcriptase (RT)-PCR analysis of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) expression levels in left ventricles of sham or AB HFD-fed mice treated with vehicle or apelin. (d) Myocardial FA oxidation, (e) glucose oxidation, (f) plasma glucose level and (g) glucose tolerance test (GTT) in vehicle- or apelin-treated HFD-fed subjected to sham or AB surgery. Results are means±s.e.m. *P<0.05; **P<0.01; ***P<0.001 vs sham-vehicle; §_P<0.05; §§_P<0.01; §§§P<0.001 vs AB-vehicle.
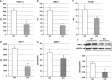
Figure 3
Mitochondrial abnormalities in heart failure linked to obesity: effects of apelin. (a) Representative electron micrographs of cardiac tissues from vehicle- or apeline-treated HFD-fed mice subjected to sham or AB for 4 weeks: M, mitochondria; Myo, myofilaments; L, lipid droplets; arrow, swelling; asterisks, structural disruption, original magnifications × 3000 or × 10 000. The ultrastructural injury in cardiac tissue from sham and AB mice treated with vehicle or apelin was evaluated by electron microscopy. (b) Quantitative analysis of mitochondrial density in heart tissues based on analysis of electron micrographs (_n_=5 animals per group). (c) Real-time PCR mtDNA/nDNA ratios (_n_=5), (d) myocardial citrate synthase activity, (e–g) myocardial expression of mitochondrial biogenesis-related genes. (i) Real-time reverse transcriptase (RT)-PCR analysis and (k) western blot analysis of Sirt3 expression in vehicle- (n_=6) or apeline-treated (n_=6) HFD-fed mice after 4 weeks of AB. *P<0.05; ***P<0.001 vs sham-vehicle; §_P<0.05; §§§_P<0.001 vs AB-vehicle.
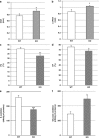
Figure 4
Cardiometabolic phenotype of apelin KO mice. Echocardiographic evaluation of (a) interventricular septum thickness (IVST), (b) LV posterior wall thickness (LVPWT), (c) fractional shortening (FS), (d) ejection fraction (EF) in WT (_n_=6) or apelin KO (_n_=7) mice. (e) Myocardial FA oxidation and (f) glucose oxidation in WT or apelin KO mice. Results are means±s.e.m. *P<0.05; **P<0.01; ***P<0.001 vs WT mice.
Figure 5
Mitochondrial biogenesis-related gene expression levels in apelin KO mice. (a–e) Real-time reverse transcriptase (RT)-PCR analysis of myocardial expression of PGC-1α, NRF-1, TFAM, CPT1 and Sirt3 in apelin KO or WT mice. (f) Western blot analysis and densitometric evaluation of Sirt3 level in apelin KO (_n_=5) or WT (_n_=5) mice. Results are means±s.e.m. *P<0.05; **P<0.01 vs WT mice.
Figure 6
Apelin-induced stimulation of Sirt3-dependent FA oxidation in isolated cardiomyocytes. (a) Effect of increasing doses of apelin (10−9–10−6 M) on FA oxidation in cardiomyocytes isolated from HFD-fed mice. (b) Dose-dependent effect of apelin (10−9–10−7 M) on Sirt3 mRNA expression at 24 h. (c) Western blot analysis of Sirt3 protein level after apelin treatment (10−7M, 24 h) in isolated cardiomyocytes. (d) Representative images of cultured cardiomyocytes after 24 h transfection with Sirt3 siRNA or siRNA negative control (Scramble) with or without apelin stimulation for 1 h. (e) Effect of apelin (10−7 M) on FA oxidation in cultured cardiomyocytes after 24 h transfection with Sirt3 siRNA or Scramble. Data are representative of three independent experiments. (f–h) Quantitative reverse transcriptase (RT)-PCR analysis of PGC-1α, TFAM and NRF-1 expression levels in cardiomyocytes after apelin stimulation (10−7 M) for 24 h. Results are means±s.e.m. (n_=3). *P<0.05; **P<0.01 vs control; §_P<0.05 vs apelin-treated Scramble.
Similar articles
- Apelin-13 administration protects against ischaemia/reperfusion-mediated apoptosis through the FoxO1 pathway in high-fat diet-induced obesity.
Boal F, Timotin A, Roumegoux J, Alfarano C, Calise D, Anesia R, Parini A, Valet P, Tronchere H, Kunduzova O. Boal F, et al. Br J Pharmacol. 2016 Jun;173(11):1850-63. doi: 10.1111/bph.13485. Epub 2016 Apr 20. Br J Pharmacol. 2016. PMID: 27005319 Free PMC article. - Short-term but not long-term high fat diet feeding protects against pressure overload-induced heart failure through activation of mitophagy.
Tan Y, Li M, Wu G, Lou J, Feng M, Xu J, Zhou J, Zhang P, Yang H, Dong L, Li J, Zhang X, Gao F. Tan Y, et al. Life Sci. 2021 May 1;272:119242. doi: 10.1016/j.lfs.2021.119242. Epub 2021 Feb 16. Life Sci. 2021. PMID: 33607155 - Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding.
Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Wright JJ, et al. Cardiovasc Res. 2009 May 1;82(2):351-60. doi: 10.1093/cvr/cvp017. Epub 2009 Jan 15. Cardiovasc Res. 2009. PMID: 19147655 Free PMC article. - Acetylation control of cardiac fatty acid β-oxidation and energy metabolism in obesity, diabetes, and heart failure.
Fukushima A, Lopaschuk GD. Fukushima A, et al. Biochim Biophys Acta. 2016 Dec;1862(12):2211-2220. doi: 10.1016/j.bbadis.2016.07.020. Epub 2016 Jul 29. Biochim Biophys Acta. 2016. PMID: 27479696 Review. - Cardiac lipid metabolism, mitochondrial function, and heart failure.
Da Dalt L, Cabodevilla AG, Goldberg IJ, Norata GD. Da Dalt L, et al. Cardiovasc Res. 2023 Aug 19;119(10):1905-1914. doi: 10.1093/cvr/cvad100. Cardiovasc Res. 2023. PMID: 37392421 Free PMC article. Review.
Cited by
- Metformin Attenuates Postinfarction Myocardial Fibrosis and Inflammation in Mice.
Loi H, Kramar S, Laborde C, Marsal D, Pizzinat N, Cussac D, Roncalli J, Boal F, Tronchere H, Oleshchuk O, Korda M, Kunduzova O. Loi H, et al. Int J Mol Sci. 2021 Aug 30;22(17):9393. doi: 10.3390/ijms22179393. Int J Mol Sci. 2021. PMID: 34502314 Free PMC article. - The Role of Apelin/Apelin Receptor in Energy Metabolism and Water Homeostasis: A Comprehensive Narrative Review.
Hu G, Wang Z, Zhang R, Sun W, Chen X. Hu G, et al. Front Physiol. 2021 Feb 10;12:632886. doi: 10.3389/fphys.2021.632886. eCollection 2021. Front Physiol. 2021. PMID: 33679444 Free PMC article. Review. - Apelin-13 administration protects against ischaemia/reperfusion-mediated apoptosis through the FoxO1 pathway in high-fat diet-induced obesity.
Boal F, Timotin A, Roumegoux J, Alfarano C, Calise D, Anesia R, Parini A, Valet P, Tronchere H, Kunduzova O. Boal F, et al. Br J Pharmacol. 2016 Jun;173(11):1850-63. doi: 10.1111/bph.13485. Epub 2016 Apr 20. Br J Pharmacol. 2016. PMID: 27005319 Free PMC article. - Inhibition of PIKfyve prevents myocardial apoptosis and hypertrophy through activation of SIRT3 in obese mice.
Tronchere H, Cinato M, Timotin A, Guitou L, Villedieu C, Thibault H, Baetz D, Payrastre B, Valet P, Parini A, Kunduzova O, Boal F. Tronchere H, et al. EMBO Mol Med. 2017 Jun;9(6):770-785. doi: 10.15252/emmm.201607096. EMBO Mol Med. 2017. PMID: 28396567 Free PMC article. - Effect of L-Carnitine Supplementation on Apelin and Apelin Receptor (Apj) Expression in Cardiac Muscle of Obese Diabetic Rats.
Ranjbar Kohan N, Nazifi S, Tabandeh MR, Ansari Lari M. Ranjbar Kohan N, et al. Cell J. 2018 Oct;20(3):427-434. doi: 10.22074/cellj.2018.5408. Epub 2018 May 15. Cell J. 2018. PMID: 29845798 Free PMC article.
References
- Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:59–113. - PubMed
- Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta. 1994;1213:263–276. - PubMed
- Van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res. 2000;45:279–293. - PubMed
- Visser FC. Imaging of cardiac metabolism using radiolabelled glucose, fatty acids and acetate. Coron Artery Dis. 2001;12 (Suppl 1:S12–S18. - PubMed
- Hendrickson SC, St, Louis JD, Lowe JE, Abdel-aleem S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol Cell Biochem. 1997;166:85–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials