BDNF is associated with SFRP1 expression in luminal and basal-like breast cancer cell lines and primary breast cancer tissues: a novel role in tumor suppression? - PubMed (original) (raw)
Comparative Study
BDNF is associated with SFRP1 expression in luminal and basal-like breast cancer cell lines and primary breast cancer tissues: a novel role in tumor suppression?
Laura Huth et al. PLoS One. 2014.
Abstract
Secreted frizzled related protein 1 (SFRP1) functions as an important inhibitor of the Wnt pathway and is a known tumor suppressor gene, which is epigenetically silenced in a variety of tumors e.g. in breast cancer. However, it is still unclear how SFRP1 exactly affects the Wnt pathway. Our aim was to decipher SFRP1 involvement in biochemical signaling in dependency of different breast cancer subtypes and to identify novel SFRP1-regulated genes. We generated SFRP1 over-expressing in vitro breast cancer models, reflecting the two major subtypes by using basal-like BT20 and luminal-like HER2-positive SKBR3 cells. DNA microarray expression profiling of these models revealed that SFRP1 expression potentially modulates Bone morphogenetic protein- and Smoothened signaling (p<0.01), in addition to the known impact on Wnt signaling. Importantly, further statistical analysis revealed that in dependency of the cancer subtype model SFRP1 may affect the canonical and non-canonical Wnt pathway (p<0.01), respectively. While SFRP1 re-expression generally mediated distinct patterns of transcriptionally induced or repressed genes in BT20 and SKBR3 cells, brain derived neurotrophic factor (BDNF) was identified as a SFRP1 induced gene in both cell lines. Although BDNF has been postulated as a putative oncogene, the co-regulation with SFRP1 indicates a potential suppressive function in breast cancer. Indeed, a positive correlation between SFRP1 and BDNF protein expression could be shown (p<0.001) in primary breast cancer samples. Moreover, TCGA dataset based analysis clearly underscores that BDNF mRNA is down-regulated in primary breast cancer samples predicting a poor prognosis of these patients. In line, we functionally provide evidence that stable BDNF re-expression in basal-like BT20 breast cancer cells blocks tumor cell proliferation. Hence, our results suggest that BDNF might rather mediate suppressive than promoting function in human breast cancer whose mode of action should be addressed in future studies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
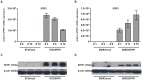
Figure 1. Generation of human breast cancer cell lines stably expressing SFRP1.
Stable clones have been generated with a full-length SFRP1 cDNA or with empty pEF6/V5 vector control. (A) Semi-quantitative real-time PCR for SFRP1 re-expression was performed after transfection in BT20 and (B) SKBR3 cells. SFRP1 mRNA was only detectable in the SFRP1 clones. (C) Western blot analysis was performed on lysates of three BT20 and (D) SKBR3 mock and of three SFRP1 clones. SFRP1 protein expression increased remarkably after transfection with a SFRP1 expression vector compared to the corresponding mock vector. β-actin was used as a loading control.
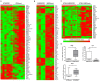
Figure 2. Microarray analysis from stable transfected cell lines BT20 and SKBR3.
Genes represented have a p value of 0.05 or less and are regulated at least ±2-fold. (A) 87 differentially expressed genes were found by comparing BT20/SFRP1 and BT20/mock cells. (B) Comparison of SKBR3/SFRP1 and SKBR3/mock cells revealed 104 differentially expressed genes. (C) By applying class comparison between SFRP1 clones (BT20 and SKBR3) and mock clones (BT20 and SKBR3) 40 differentially expressed genes were discovered. (D) Validation of SFRP1 target genes in SKBR3 and BT20 model system. Semi-quantitative real-time PCR was performed for each target gene in the particular in vitro model. BDNF mRNA levels increased in SKBR3/SFRP1 clones compared to the mock controls (p<0.05). In contrast LY96 mRNA was up-regulated in SKBR3 mock clones (p<0.05). BT20 cells showed an increase of BDNF mRNA levels after SFRP1 re-expression (p<0.05).
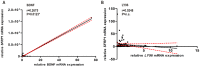
Figure 3. Correlation analysis of mRNA expression in human breast cancer tissues.
(A) By comparing the BDNF and SFRP1 mRNA expression of 87 tumor samples a significant correlation was found (Spearman coefficient, p<0.05). (B) A correlation between LY96 and SFRP1 mRNA expression in 86 human breast cancer tissues could not be detected. n.s.: not significant.
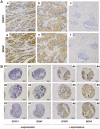
Figure 4. Analysis of SFRP1 and BDNF expression in human breast tissues.
(A) Immunohistochemical staining of human breast tissues is shown exemplarily. SFRP1 and BDNF protein expression of tissue samples from the same patient (a and d, b and e) is shown. c and f: negative controls of SFRP1 and BDNF immunohistochemical staining, respectively. Scale bar: 100 µm. (B) In the left columns three tissues (patient #1 - #3) exhibit no or weak staining of SFRP1 and BDNF whereas the right columns represent three tissues (patient #4 - #6) that have a strong SFRP1 and BDNF protein expression. These images illustrate exemplarily the correlation of SFRP1 and BDNF protein expression in vivo. Scale bars: 100 µm.
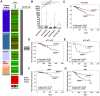
Figure 5. BDNF gene expression is associated with longer recurrence-free survival in human breast cancer of public TCGA data sets .
(A) BDNF expression in breast tumor samples. Red: high expression, black: mean expression and green: low expression. Left panel: clinical data. Middle panel: BDNF mRNA expression. Right panel: sample type (dark grey: primary tumor; white: solid normal tissues; based on TCGA Ilumina platform, n = 1032 samples). (B) BDNF expression in relation to tumors stratified by subtypes . ns: not significant, *p<0.05, ***p<0.001. (C–G) Kaplan-Meier analysis of the TCGA data set illustrating RFS of patients with high BDNF (red curve) compared to reduced BDNF expression (black curve) in (C) all, (D) pT1-pT2 (E) pT3-pT4 (F) nodal-negative (pN0) or (G) nodal-postive (pN+) breast cancer patients. Vertical lines: censored cases.
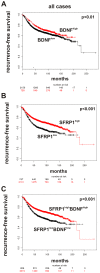
Figure 6. The clinical impact of SFRP1/BDNF gene expression in human breast cancer.
Using the KMPLOT data set an association between unfavorable clinical outcome for breast cancer patients and (A) SFRP1 expression, as well as (B) BDNF expression and (C) a combination of abundant SFRP1/BDNF expression was observed.
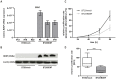
Figure 7. BDNF re-expression mediates reduced cell proliferation in BT20 breast cancer cells.
(A) Stable cell clones with a full-length cDNA of BDNF show abundant re-expression of BDNF mRNA while empty pT-Rex-DEST30 vector controls completely lack BDNF mRNA. (B) In concordance, mock clones are negative for BDNF protein whereas the 25 kDa BDNF protein is strongly expressed in stable BDNF clones. (C) XTT assay was performed at four subsequent time points. The baseline level at 24 h for each clone was set to 1. A slight decrease in cell proliferation (58.4%) was observed in the stable BDNF clones. (D) Proliferation is significantly (p<0.001) reduced in BT20 breast cancer cells re-expressing BDNF.
Similar articles
- Role of Secreted Frizzled-Related Protein 1 in Early Mammary Gland Tumorigenesis and Its Regulation in Breast Microenvironment.
Clemenceau A, Diorio C, Durocher F. Clemenceau A, et al. Cells. 2020 Jan 14;9(1):208. doi: 10.3390/cells9010208. Cells. 2020. PMID: 31947616 Free PMC article. Review. - WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth.
Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE. Matsuda Y, et al. Breast Cancer Res. 2009;11(3):R32. doi: 10.1186/bcr2317. Epub 2009 May 27. Breast Cancer Res. 2009. PMID: 19473496 Free PMC article. - Methylation-associated silencing of SFRP1 with an 8p11-12 amplification inhibits canonical and non-canonical WNT pathways in breast cancers.
Yang ZQ, Liu G, Bollig-Fischer A, Haddad R, Tarca AL, Ethier SP. Yang ZQ, et al. Int J Cancer. 2009 Oct 1;125(7):1613-21. doi: 10.1002/ijc.24518. Int J Cancer. 2009. PMID: 19569235 Free PMC article. - Epigenetics of SFRP1: The Dual Roles in Human Cancers.
Baharudin R, Tieng FYF, Lee LH, Ab Mutalib NS. Baharudin R, et al. Cancers (Basel). 2020 Feb 14;12(2):445. doi: 10.3390/cancers12020445. Cancers (Basel). 2020. PMID: 32074995 Free PMC article. Review.
Cited by
- Role of Secreted Frizzled-Related Protein 1 in Early Mammary Gland Tumorigenesis and Its Regulation in Breast Microenvironment.
Clemenceau A, Diorio C, Durocher F. Clemenceau A, et al. Cells. 2020 Jan 14;9(1):208. doi: 10.3390/cells9010208. Cells. 2020. PMID: 31947616 Free PMC article. Review. - Effects of Chinese medicinal herbs on expression of brain-derived Neurotrophic factor (BDNF) and its interaction with human breast cancer MDA-MB-231 cells and endothelial HUVECs.
Chiu JH, Chen FP, Tsai YF, Lin MT, Tseng LM, Shyr YM. Chiu JH, et al. BMC Complement Altern Med. 2017 Aug 12;17(1):401. doi: 10.1186/s12906-017-1909-7. BMC Complement Altern Med. 2017. PMID: 28800782 Free PMC article. - Breast Cancer and Microcalcifications: An Osteoimmunological Disorder?
Clemenceau A, Michou L, Diorio C, Durocher F. Clemenceau A, et al. Int J Mol Sci. 2020 Nov 15;21(22):8613. doi: 10.3390/ijms21228613. Int J Mol Sci. 2020. PMID: 33203195 Free PMC article. Review. - Potential biomarkers of ductal carcinoma in situ progression.
Dettogni RS, Stur E, Laus AC, da Costa Vieira RA, Marques MMC, Santana IVV, Pulido JZ, Ribeiro LF, de Jesus Parmanhani N, Agostini LP, Dos Reis RS, de Vargas Wolfgramm Dos Santos E, Alves LNR, Garcia FM, Santos JA, do Prado Ventorim D, Reis RM, Louro ID. Dettogni RS, et al. BMC Cancer. 2020 Feb 12;20(1):119. doi: 10.1186/s12885-020-6608-y. BMC Cancer. 2020. PMID: 32050925 Free PMC article. - Crucial microRNAs and genes of human primary breast cancer explored by microRNA-mRNA integrated analysis.
Yang Y, Xing Y, Liang C, Hu L, Xu F, Chen Y. Yang Y, et al. Tumour Biol. 2015 Jul;36(7):5571-9. doi: 10.1007/s13277-015-3227-3. Epub 2015 Feb 14. Tumour Biol. 2015. PMID: 25680412
References
- Turashvili G, Bouchal J, Burkadze G, Kolar Z (2006) Wnt signaling pathway in mammary gland development and carcinogenesis. Pathobiology 73: 213–223. - PubMed
- Janssen KP, Alberici P, Fsihi H, Gaspar C, Breukel C, et al. (2006) APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 131: 1096–1109. - PubMed
- Nusse R (2005) Wnt signaling in disease and in development. Cell Res 15: 28–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by DFG grant DA329/3-1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous