Targeting autophagy in breast cancer - PubMed (original) (raw)
Review
Targeting autophagy in breast cancer
Paola Maycotte et al. World J Clin Oncol. 2014.
Abstract
Macroautophagy (referred to as autophagy here) is an intracellular degradation pathway enhanced in response to a variety of stresses and in response to nutrient deprivation. This process provides the cell with nutrients and energy by degrading aggregated and damaged proteins as well as compromised organelles. Since autophagy has been linked to diverse diseases including cancer, it has recently become a very interesting target in breast cancer treatment. Indeed, current clinical trials are trying to use chloroquine or hydroxychloroquine, alone or in combination with other drugs to inhibit autophagy during breast cancer therapy since chemotherapy and radiation, regimens that are used to treat breast cancer, are known to induce autophagy in cancer cells. Importantly, in breast cancer, autophagy has been involved in the development of resistance to chemotherapy and to anti-estrogens. Moreover, a close relationship has recently been described between autophagy and the HER2 receptor. Here, we discuss some of the recent findings relating autophagy and cancer with a particular focus on breast cancer therapy.
Keywords: Autophagy; Breast cancer; Cancer; Chemotherapy; Estrogen receptor; HER2; Radiation; Triple negative breast cancer.
Figures
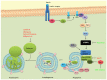
Figure 1
The autophagic process and some of its major regulators. The phosphatidylinositol 3-kinase (PI3K) pathway is triggered by the binding of insulin or growth factors to the insulin receptor, activating PI3K. Activated PI3K converts PIP2 to PIP3 which then recruits PDK1 and Akt to the plasma membrane. Activated Akt then phosphorylates and inactivates TSC 1/2, leading to the activation of Rheb and mammalian target of rapamycin complex 1 (mTORC1). Under nutrient-rich conditions, mTORC1 suppresses autophagy by phosphorylating and inhibiting the ULK1 complex. Under starvation conditions or rapamycin treatment, mTOR dissociates from the ULK1 complex and autophagy gets activated. ULK1 is also directly phosphorylated and activated by the AMP-activated protein kinase (AMPK) in response to energy restriction. The autophagic process involves the degradation of cytosolic proteins and organelles in the lysosomes via autophagosomal delivery. Two complexes regulate the formation of the phagophore, the ULK1 complex and the beclin 1-VPS34 (class III PI3K) complex. The elongation of the autophagosomal membrane is mediated by two ubiquitin-like protein conjugation systems: ATG12-ATG5 and ATG8/LC3. LC3 can additionally recruit adaptor proteins such as p62 to autophagosomes mediating selective autophagy of cellular structures, protein aggregates and microorganisms. LC3II (LC3 bound to phosphatidylethanolamine) is recruited to both the inner and outer autophagosomal membrane. Autophagosomes fuse with lysosomes to form autolysosomes where they are degraded together with their luminal content. Pharmacological modulators of autophagy are labeled according to their effect on autophagy. Red: Inhibits autophagy; Green: Induces autophagy.
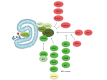
Figure 2
Beclin 1, its protein interactions and its role in autophagy. Beclin 1 has been found to form part of different class III phosphatidylinositol 3-kinase (PI3K) complexes. Each complex consists of beclin 1, Vps34, Vps15 and Ambra1. ATG14L activates the complex and induces the formation of autophagosomes. UVRAG and ATG14L are present in mutually exclusive class III PI3K complexes and UVRAG and Bif-1 have been shown to activate the complex. UVRAG has also been shown to function in autophagosome maturation and endocytic trafficking possibly independent from its interaction with beclin 1. Rubicon has also been shown to bind beclin 1 and can inhibit autophagosome formation and maturation. TAB1/2, two upstream activators of the TAK1-IKK signaling pathway interact with beclin 1 and their dissociation seems to be necessary for autophagy induction. GAPR-1 can also bind beclin 1 and inhibit autophagy probably through beclin 1 tethering in the Golgi apparatus. Bcl-2/Bcl-XL can bind beclin 1 and inhibit autophagy. JNK1 phosphorylates Bcl-2 while DAPK phosphorylates beclin 1 and disrupt their interaction. Additionally, BH3-only proteins (tBid, Bad, BNIP3) or BH3 mimetics (ABT737) bind Bcl-2 and release beclin 1. Beclin 1 has also been found to be a substrate of caspases-3, 7 and 8 during apoptosis, in which cleavage of beclin 1 suppresses autophagy. Other beclin 1 interacting proteins that induce autophagy are PINK1 and VMP1. TRAF6 and USP13 have been shown to regulate beclin 1 ubiquitination. Survivin, an anti-apoptotic protein can also bind beclin 1 and regulate TRAIL induced apoptosis. NAF-1, a component of the IP3 receptor complex contributes to the interaction of Bcl-2 with beclin 1 at the ER[32,99-104]. Proteins and drugs shown are color-coded according to their effect on autophagy. Red: Inhibits autophagy; Green: Induces autophagy; Yellow: Unknown.
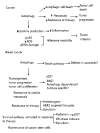
Figure 3
Autophagy in cancer and breast cancer. Different roles have been described for autophagy in cancer. Autophagy can limit tumor initiation by decreasing inflammation and genome instability. Once a tumor is established, autophagy can induce tumor progression by facilitating metastasis and resistance to therapy. On the other hand, it has also been proposed that autophagy could function as a cell death process that could be induced during therapy. In breast cancer, although some evidence suggests that autophagic cell death occurs in apoptosis defective cells, most of the evidence in the literature suggests a tumor promoting role for autophagy. Autophagy has been shown to promote tumorigenesis, tumor proliferation and progression. This could depend on the p53 or RAS mutation status of the cells. Also, if breast cancer is an autophagy-dependent disease remains to be determined as well as if this dependence is subtype specific. Autophagy has also been involved in resistance to anoikis, facilitation of metastasis, resistance to therapy and maintenance of breast cancer stem cells. Moreover, when cancer cells are made resistant to the therapies shown in the figure, they show increased autophagy and inhibition of autophagy reverts this resistance. Therapies that are known to induce autophagy in breast cancer are also shown. With these treatments, autophagy inhibition has been shown to increase cell death. Regarding radiation, results are controversial and the role of autophagy could depend on the p53 status of the cell.
Similar articles
- The role of autophagy in HER2-targeted therapy.
Janser FA, Tschan MP, Langer R. Janser FA, et al. Swiss Med Wkly. 2019 Oct 27;149:w20138. doi: 10.4414/smw.2019.20138. eCollection 2019 Oct 7. Swiss Med Wkly. 2019. PMID: 31656036 Review. - Autophagy and endocrine resistance in breast cancer.
Cook KL, Shajahan AN, Clarke R. Cook KL, et al. Expert Rev Anticancer Ther. 2011 Aug;11(8):1283-94. doi: 10.1586/era.11.111. Expert Rev Anticancer Ther. 2011. PMID: 21916582 Free PMC article. Review. - Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells.
Rao R, Balusu R, Fiskus W, Mudunuru U, Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P, Bhalla KN. Rao R, et al. Mol Cancer Ther. 2012 Apr;11(4):973-83. doi: 10.1158/1535-7163.MCT-11-0979. Epub 2012 Feb 24. Mol Cancer Ther. 2012. PMID: 22367781 - Therapeutic Targeting of Autophagy.
Towers CG, Thorburn A. Towers CG, et al. EBioMedicine. 2016 Dec;14:15-23. doi: 10.1016/j.ebiom.2016.10.034. Epub 2016 Oct 23. EBioMedicine. 2016. PMID: 28029600 Free PMC article. Review. - Nanomaterials and autophagy: new insights in cancer treatment.
Panzarini E, Inguscio V, Tenuzzo BA, Carata E, Dini L. Panzarini E, et al. Cancers (Basel). 2013 Mar 21;5(1):296-319. doi: 10.3390/cancers5010296. Cancers (Basel). 2013. PMID: 24216709 Free PMC article.
Cited by
- Autophagy Modulation in Therapeutic Strategy of Breast Cancer Drug Resistance.
Wang M, Liu M, Yang C, Hu Y, Liao X, Liu Q. Wang M, et al. J Cancer. 2024 Aug 19;15(16):5462-5476. doi: 10.7150/jca.97775. eCollection 2024. J Cancer. 2024. PMID: 39247603 Free PMC article. Review. - Autophagy as a Therapeutic Target in Breast Tumors: The Cancer stem cell perspective.
Raza S, Siddiqui JA, Srivastava A, Chattopadhyay N, Sinha RA, Chakravarti B. Raza S, et al. Autophagy Rep. 2024 Jun 24;3(1):27694127.2024.2358648. doi: 10.1080/27694127.2024.2358648. eCollection 2024 Dec 31. Autophagy Rep. 2024. PMID: 39006309 - Autophagy Deregulation in HIV-1-Infected Cells Increases Extracellular Vesicle Release and Contributes to TLR3 Activation.
DeMarino C, Cowen M, Williams A, Khatkar P, Abulwerdi FA, Henderson L, Denniss J, Pleet ML, Luttrell DR, Vaisman I, Liotta LA, Steiner J, Le Grice SFJ, Nath A, Kashanchi F. DeMarino C, et al. Viruses. 2024 Apr 20;16(4):643. doi: 10.3390/v16040643. Viruses. 2024. PMID: 38675983 Free PMC article. - Evaluation of Beclin1 and mTOR genes and p62 protein expression in breast tumor tissues of Iranian patients.
Adelipour M, Naghashpour M, Roshanazadeh MR, Chenaneh H, Mohammadi A, Pourangi P, Miri SR, Zahedi A, Haghighatnezhad M, Golabi S. Adelipour M, et al. Mol Biol Res Commun. 2024;13(1):11-19. doi: 10.22099/mbrc.2023.47597.1837. Mol Biol Res Commun. 2024. PMID: 38164366 Free PMC article. - RL2 Enhances the Elimination of Breast Cancer Cells by Doxorubicin.
Wohlfromm F, Seyrek K, Ivanisenko N, Troitskaya O, Kulms D, Richter V, Koval O, Lavrik IN. Wohlfromm F, et al. Cells. 2023 Dec 6;12(24):2779. doi: 10.3390/cells12242779. Cells. 2023. PMID: 38132099 Free PMC article.
References
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. - PubMed
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous