Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation - PubMed (original) (raw)
Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation
Yvette C Wong et al. Proc Natl Acad Sci U S A. 2014.
Abstract
Mitophagy is a cellular quality control pathway in which the E3 ubiquitin ligase parkin targets damaged mitochondria for degradation by autophagosomes. We examined the role of optineurin in mitophagy, as mutations in optineurin are causative for amyotrophic lateral sclerosis (ALS) and glaucoma, diseases in which mitochondrial dysfunction has been implicated. Using live cell imaging, we demonstrate the parkin-dependent recruitment of optineurin to mitochondria damaged by depolarization or reactive oxygen species. Parkin's E3 ubiquitin ligase activity is required to ubiquitinate outer mitochondrial membrane proteins, allowing optineurin to stably associate with ubiquitinated mitochondria via its ubiquitin binding domain; in the absence of parkin, optineurin transiently localizes to damaged mitochondrial tips. Following optineurin recruitment, the omegasome protein double FYVE-containing protein 1 (DFCP1) transiently localizes to damaged mitochondria to initialize autophagosome formation and the recruitment of microtubule-associated protein light chain 3 (LC3). Optineurin then induces autophagosome formation around damaged mitochondria via its LC3 interaction region (LIR) domain. Depletion of endogenous optineurin inhibits LC3 recruitment to mitochondria and inhibits mitochondrial degradation. These defects are rescued by expression of siRNA-resistant wild-type optineurin, but not by an ALS-associated mutant in the ubiquitin binding domain (E478G), or by optineurin with a mutation in the LIR domain. Optineurin and p62/SQSTM1 are independently recruited to separate domains on damaged mitochondria, and p62 is not required for the recruitment of either optineurin or LC3 to damaged mitochondria. Thus, our study establishes an important role for optineurin as an autophagy receptor in parkin-mediated mitophagy and demonstrates that defects in a single pathway can lead to neurodegenerative diseases with distinct pathologies.
Keywords: Parkinson's disease; amyotrophic lateral sclerosis; autophagosome; mitophagy; optineurin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
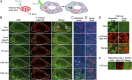
Fig. 1.
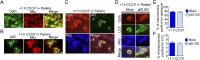
Optineurin transiently associates with damaged mitochondria in the absence of parkin. (A) One hour of CCCP (20 µM) treatment of HeLa cells causes mitochondrial fragmentation. Optineurin puncta (GFP-Optn) in control cells do not associate with mitochondria (DsRed2-mito) (yellow arrows). In HeLa cells in the absence of parkin, CCCP-induced mitochondrial damage causes optineurin puncta to transiently associate (white arrows) with the tips of fragmented mitochondria (arrowheads). (B) Optineurin preferentially localizes to damaged mitochondria immediately following CCCP treatment. (C) Time series of optineurin recruitment from the cytosol (pink arrows) to a mitochondrial tip (white arrows, optineurin puncta; arrowheads, mitochondria). (D) Example of optineurin (arrow) localized to a mitochondrial tip (arrowhead). (E and F) After 1-h CCCP treatment of HeLa cells, optineurin preferentially localizes to the mitochondrial tip for ∼30 s. [Scale bar, (A, C, and D) 1 µm.] Values represent means ± SEM; ***P < 0.001.
Fig. 2.
Parkin ubiquitination stabilizes optineurin recruitment to the outer membrane of damaged mitochondria. (A) Confocal image of a HeLa cell expressing DsRed2-mito and YFP-parkin after 1 h of CCCP treatment. Parkin is recruited to the outer membrane of damaged mitochondria, seen as parkin rings around spherical mitochondrial fragments within a single z plane, with corresponding line scan (Right). (B) Two hours of CCCP treatment causes aggregation of parkin-labeled mitochondria. (C) The majority of mitochondria are parkin-positive after 1 h of CCCP treatment. (D) Confocal image of a HeLa cell expressing parkin, GFP-Optn, and DsRed2-mito treated with CCCP for 1 h. Optineurin is stably recruited to the surface of damaged mitochondria in the presence of parkin. (E) Optineurin localization on the outer membrane of damaged mitochondria with corresponding line scan (Right). (F) Optineurin recruitment lags parkin recruitment. (G) Optineurin is recruited to the majority of mitochondria following 2 h of CCCP treatment. (H) Immunostaining of endogenous optineurin in 1 h of CCCP-treated HeLa cells expressing parkin and BFP-mito (pseudocolored red) with corresponding line scan (Lower). Optineurin is recruited to the outer membrane of damaged mitochondria. (I) Confocal time series of a CCCP-treated HeLa cell expressing mCherry-Optn, YFP-parkin, and BFP-mito showing optineurin recruitment only occurs following parkin recruitment to damaged mitochondria. An optineurin puncta is first localized to the surface of parkin-labeled mitochondria and gradually grows into a ring. (J) CCCP-treated HeLa cells expressing GFP-Optn, mCherry-parkin, and BFP-mito. Confocal image and corresponding line scan (Lower) of damaged mitochondria with both optineurin and parkin localized to its outer membrane. (K) Both optineurin and parkin localize to mitochondria following 2 h of CCCP treatment. (L) A catalytically inactive Parkinson’s disease-associated parkin mutant T240R is unable to recruit optineurin after either 1- or 3-h CCCP. [Scale bar, (A, B, D, G, and K) 10 µm, (Insets in A, B, D, and G) 1 µm, and (E, H, I, J, and L) 1 µm.] Values represent means ± SEM; **P < 0.01, ***P < 0.001.
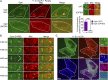
Fig. 3.
Optineurin is dynamically recruited to damaged mitochondria via parkin. (A, Schematic) Illumination of mitochondrial-targeted mito-KR by 561-nm light leads to the generation of ROS within the mitochondrial matrix. Illumination of a defined region within the cell (yellow box) allows for spatiotemporally controlled damage of a specific mitochondrial population (black mitochondria). (B) Confocal image of a HeLa cell expressing parkin, GFP-Optn, mito-KR, and BFP-mito. A defined region was illuminated (yellow box) leading to Mito-KR bleaching and induced mitochondrial damage. Optineurin is recruited to the surface of damaged mitochondria beginning ∼25 min after bleaching. (C and D) Optineurin recruitment is spatially restricted to mitochondria near the bleached region rather than to a distal unbleached region of the cell. (E) Optineurin remains localized to mitochondria in the bleached area 1 h after bleaching. (F) Optineurin is not recruited to damaged mitochondria in a HeLa cell not expressing exogenous parkin, demonstrating that optineurin recruitment to spatiotemporally restricted damaged mitochondria also requires parkin. [Scale bar, (B) 10 µm and (C_–_F) 1 µm.]
Fig. 4.
Optineurin stably associates with damaged ubiquitinated mitochondria via its UBAN domain and is disrupted by an ALS-linked mutation. (A) Confocal image of a CCCP-treated HeLa cell expressing parkin, BFP-mito (pseudocolored red), and either wild-type mCherry-Optn or an ALS-associated UBAN mutant mCherry–Optn-E478G that has deficient ubiquitin binding (optn-E478G) (pseudocolored green). Wild-type optn shows clear recruitment to the surface of damaged mitochondria (white arrows on magnified image) after 1-h CCCP, whereas optn-E478G does not stably associate with damaged mitochondria. Quantification of optineurin recruitment to damaged mitochondria showing that wild-type optineurin is preferentially recruited and stabilized on mitochondria compared with the E478G mutant. (B) Time lapse showing optn-E478G puncta are transiently recruited to the outer surface of damaged mitochondria (white arrows). However, optn-E478G does not remain stably associated, resulting in mitochondria not labeled with optn-E478G (yellow arrows). (C) Optn-E478G remains cytosolic even after 1.5 h of CCCP treatment and does not localize to parkin-positive mitochondria (yellow arrows). [Scale bar, (A) 10 µm, (C) 5 µm, (Insets in A and C) 1 µm, and (B) 1 µm.] Values represent means ± SEM; ***P < 0.001.
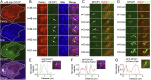
Fig. 5.
DFCP1 omegasome formation occurs after parkin/optineurin recruitment to damaged mitochondria. (A) HeLa cell expressing BFP-mito, mCherry-parkin, and the omegasome marker GFP-DFCP1 treated with CCCP for 45 min. DFCP1 puncta are transiently recruited to parkin-positive mitochondria. (B) Time-lapse images of boxed area in A following CCCP treatment showing DFCP1 puncta recruited to parkin-labeled mitochondria for ∼3 min before leaving (arrows). (C) Two examples of a DFCP1 puncta gradually forming on an optineurin-labeled mitochondria over ∼3 min and then disappearing (white arrows and pink arrows). (D) Example of DFCP1 recruitment to the surface of an optineurin-labeled mitochondria and later forming DFCP1 tubules (yellow arrows) that extend outward. (E–G) Line scans showing DFCP1 localization with parkin and optineurin on the outer membrane of damaged mitochondria, from HeLa cells expressing GFP-DFCP1 with BFP-mito, mCherry-parkin, or mCherry-Optn. [Scale bar, (A) 5 µm and (B–D) 1 µm.]
Fig. 6.
Optineurin recruits LC3 autophagosomes to damaged mitochondria via its LIR domain. (A and B) Confocal images of a HeLa cell expressing parkin, mCherry-Optn, and the autophagosome marker GFP-LC3 treated with CCCP for 1 h. Optineurin is recruited to the surface of damaged mitochondria via parkin expression. LC3 autophagosomes dynamically form around optineurin-labeled damaged mitochondria (white arrows). Not all optineurin-labeled mitochondria are LC3-positive at this time point (yellow arrows). (C) Increased recruitment of LC3 to optineurin-labeled mitochondria after 1.5 h of CCCP treatment (white arrows). (D–F) HeLa cells expressing parkin, GFP-LC3, and BFP-mito (pseudocolored red). Formation of LC3 autophagosomes around damaged mitochondria (white arrows) in control cells with endogenous levels of optineurin (Mock) after 1 h of CCCP treatment is inhibited by siRNA knockdown (KD) of endogenous optineurin (Optn KD). (G) HeLa cell expressing parkin, mCherry-Optn, GFP-LC3, and BFP-mito after 1 h of CCCP treatment showing accelerated LC3 autophagosome engulfment of optineurin-labeled mitochondria in cells with increased optineurin expression (white arrows). (H and I) Expression of ALS-associated ubiquitin binding-deficient mCherry–Optn-E478G inhibits LC3 recruitment to mitochondria (white arrows). (J–L) Expression of LC3 binding-deficient mCherry_–_Optn-F178A that localizes to damaged mitochondria inhibits LC3 recruitment to mitochondria (white arrows). (M) Optineurin depletion disrupts LC3 autophagosome formation around mitochondria and is rescued by siRNA-resistant wild-type optineurin, but not siRNA-resistant optineurin E478G or F178A. [Scale bar, (A) 5 µm and (B–E, G, H, and J) 1 µm.] Values represent means ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001. N.S., not significant.
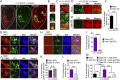
Fig. 7.
Optineurin and p62 localize to different domains on damaged mitochondria and have distinct roles in mitophagy. (A) GFP-Optn is recruited to the entire surface of damaged mitochondria after 1-h CCCP in parkin-expressing HeLa cells. (B) GFP-p62 preferentially localizes to the domains between adjacent mitochondria and p62 expression accelerates mitochondrial aggregation after 1-h CCCP. (C) mCherry-Optn and GFP-p62 localize to different domains on damaged mitochondria. (D–F) p62 depletion by siRNA results in elongated mitochondria but does not inhibit optineurin or LC3 recruitment to mitochondria after 1-h CCCP (D, Right). [Scale bar, (A–D) 1 µm.] Values represent means ± SEM.
Fig. 8.
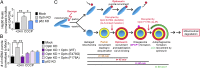
Optineurin is an autophagy receptor for damaged mitochondria and regulates mitochondrial degradation. (A) Quantification of Hsp60 by immunoblot after 24-h CCCP in parkin-expressing HeLa cells normalized to control cells and loading control GAPDH. Optineurin siRNA knockdown (KD), but not p62 siRNA KD, leads to increased Hsp60 mitochondrial matrix protein content, indicating inefficient mitochondrial degradation. (B) Quantification of mtDNA by immunofluorescence after 24-h CCCP in parkin-expressing HeLa cells. Optineurin siRNA KD, but not p62 siRNA KD, results in the accumulation of inefficiently degraded mitochondria. siRNA-resistant wild-type optineurin, but not siRNA-resistant optineurin E478G or F178A, rescues efficient clearance of damaged mitochondria. (C, Model) In the absence of parkin, optineurin puncta transiently localize to the tips of damaged mitochondria but do not remain stably associated. In the presence of parkin, parkin is first recruited to the outer membrane of damaged mitochondria, followed by optineurin recruitment via its UBAN domain to parkin-ubiquitinated mitochondria. Next, the omegasome protein DFCP1 is transiently recruited to optineurin-labeled mitochondria, marking the initial site of autophagosome formation. Optineurin then recruits LC3 to mitochondria via its LIR domain, leading to autophagosome engulfment and mitochondrial degradation. A mutation in the UBAN domain (ALS-associated E478G) disrupts optineurin recruitment, whereas a mutation in the LIR domain (F178A) disrupts LC3 recruitment. Timeline (Lower) indicates approximate half-time for each step of the parkin–optineurin–DFCP1–LC3 pathway after induced mitochondrial damage. Values represent means ± SEM; *P < 0.05, **P < 0.01.
Similar articles
- Temporal dynamics of PARK2/parkin and OPTN/optineurin recruitment during the mitophagy of damaged mitochondria.
Wong YC, Holzbaur EL. Wong YC, et al. Autophagy. 2015;11(2):422-4. doi: 10.1080/15548627.2015.1009792. Autophagy. 2015. PMID: 25801386 Free PMC article. - p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both.
Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. Narendra D, et al. Autophagy. 2010 Nov;6(8):1090-106. doi: 10.4161/auto.6.8.13426. Autophagy. 2010. PMID: 20890124 Free PMC article. - Dynamic recruitment and activation of ALS-associated TBK1 with its target optineurin are required for efficient mitophagy.
Moore AS, Holzbaur EL. Moore AS, et al. Proc Natl Acad Sci U S A. 2016 Jun 14;113(24):E3349-58. doi: 10.1073/pnas.1523810113. Epub 2016 May 31. Proc Natl Acad Sci U S A. 2016. PMID: 27247382 Free PMC article. - Role of Optineurin in the Mitochondrial Dysfunction: Potential Implications in Neurodegenerative Diseases and Cancer.
Weil R, Laplantine E, Curic S, Génin P. Weil R, et al. Front Immunol. 2018 Jun 19;9:1243. doi: 10.3389/fimmu.2018.01243. eCollection 2018. Front Immunol. 2018. PMID: 29971063 Free PMC article. Review. - Two different axes CALCOCO2-RB1CC1 and OPTN-ATG9A initiate PRKN-mediated mitophagy.
Yamano K, Youle RJ. Yamano K, et al. Autophagy. 2020 Nov;16(11):2105-2107. doi: 10.1080/15548627.2020.1815457. Epub 2020 Sep 7. Autophagy. 2020. PMID: 32892694 Free PMC article. Review.
Cited by
- Mitochondrial quality control: Cell-type-dependent responses to pathological mutant mitochondrial DNA.
Malena A, Pantic B, Borgia D, Sgarbi G, Solaini G, Holt IJ, Spinazzola A, Perissinotto E, Sandri M, Baracca A, Vergani L. Malena A, et al. Autophagy. 2016 Nov;12(11):2098-2112. doi: 10.1080/15548627.2016.1226734. Epub 2016 Sep 14. Autophagy. 2016. PMID: 27627835 Free PMC article. - Morphological multiparameter filtration and persistent homology in mitochondrial image analysis.
Chung YM, Hu CS, Sun E, Tseng HC. Chung YM, et al. PLoS One. 2024 Sep 20;19(9):e0310157. doi: 10.1371/journal.pone.0310157. eCollection 2024. PLoS One. 2024. PMID: 39302926 Free PMC article. - Autophagy and ALS: mechanistic insights and therapeutic implications.
Chua JP, De Calbiac H, Kabashi E, Barmada SJ. Chua JP, et al. Autophagy. 2022 Feb;18(2):254-282. doi: 10.1080/15548627.2021.1926656. Epub 2021 May 31. Autophagy. 2022. PMID: 34057020 Free PMC article. Review. - ALS Genetics: Gains, Losses, and Implications for Future Therapies.
Kim G, Gautier O, Tassoni-Tsuchida E, Ma XR, Gitler AD. Kim G, et al. Neuron. 2020 Dec 9;108(5):822-842. doi: 10.1016/j.neuron.2020.08.022. Epub 2020 Sep 14. Neuron. 2020. PMID: 32931756 Free PMC article. Review. - Parkin and mitophagy in cancer.
Bernardini JP, Lazarou M, Dewson G. Bernardini JP, et al. Oncogene. 2017 Mar;36(10):1315-1327. doi: 10.1038/onc.2016.302. Epub 2016 Sep 5. Oncogene. 2017. PMID: 27593930 Review.
References
- Valente EM, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. - PubMed
- Kitada T, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous