Consensus paper: radiological biomarkers of cerebellar diseases - PubMed (original) (raw)
doi: 10.1007/s12311-014-0610-3.
Stuart Currie, M Hadjivassiliou, Nigel Hoggard, Allison Jack, Andrea P Jackowski, Mario Mascalchi, Cecilia Parazzini, Kathrin Reetz, Andrea Righini, Jörg B Schulz, Alessandra Vella, Sara Jane Webb, Christophe Habas
Affiliations
- PMID: 25382714
- PMCID: PMC4929983
- DOI: 10.1007/s12311-014-0610-3
Consensus paper: radiological biomarkers of cerebellar diseases
Leonardo Baldarçara et al. Cerebellum. 2015 Apr.
Abstract
Hereditary and sporadic cerebellar ataxias represent a vast and still growing group of diseases whose diagnosis and differentiation cannot only rely on clinical evaluation. Brain imaging including magnetic resonance (MR) and nuclear medicine techniques allows for characterization of structural and functional abnormalities underlying symptomatic ataxias. These methods thus constitute a potential source of radiological biomarkers, which could be used to identify these diseases and differentiate subgroups of them, and to assess their severity and their evolution. Such biomarkers mainly comprise qualitative and quantitative data obtained from MR including proton spectroscopy, diffusion imaging, tractography, voxel-based morphometry, functional imaging during task execution or in a resting state, and from SPETC and PET with several radiotracers. In the current article, we aim to illustrate briefly some applications of these neuroimaging tools to evaluation of cerebellar disorders such as inherited cerebellar ataxia, fetal developmental malformations, and immune-mediated cerebellar diseases and of neurodegenerative or early-developing diseases, such as dementia and autism in which cerebellar involvement is an emerging feature. Although these radiological biomarkers appear promising and helpful to better understand ataxia-related anatomical and physiological impairments, to date, very few of them have turned out to be specific for a given ataxia with atrophy of the cerebellar system being the main and the most usual alteration being observed. Consequently, much remains to be done to establish sensitivity, specificity, and reproducibility of available MR and nuclear medicine features as diagnostic, progression and surrogate biomarkers in clinical routine.
Figures
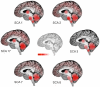
Fig. 1
Schematic representation of the main sites of atrophy (in red) observed in SCA 1, 2, 3, 6, 7 and 17 (A medulla oblongata; B pons, C cerebellum, D basal ganglia, E cerebral cortex) on sagittal view of the brain
Fig. 2
a–l Diagnostic biomarkers of recessive and X-linked inherited ataxias on conventional MRI. Axial T1 weighted image (a) showing symmetrically thickened and parallel oriented superior cerebellar peduncles (black arrowheads) creating the “Molar Tooth” sign characteristic of Joubert syndrome and related disorders. Note also the small and dysmorphic vermis. Sagittal T1-weighted image (b) shows atrophy of the medulla and cervical spinal cord (arrow) in a case of Friedreich ataxia (FRDA). Axial T2*-weighted gradient echo image (c) confirms the decreased size of the cervical cord cross-section in the same disease and demonstrates symmetric hyperintensity of the posterior (white arrowhead) and lateral (black arrowheads) columns of the spinal cord (both images reprinted with permission from ref. [1]). Sagittal T1-weighted image (d) demonstrates widened vermian sulci in a patient with ataxia telangectasia (AT) consistent with a macroscopic pattern of cerebellar cortical atrophy (image reprinted with permission from ref. [1]). Axial T2*-weighted gradient echo image (e) demonstrates multiple hypointense dots in the centrum semiovalis WM consistent with capillary telangectasias in another patient with AT (image reprinted with permission from ref. [128]). Axial (f) and sagittal (g) T2-weighted images in a patient with recessive spastic ataxia of Charlevoix-Saguenay (ARSA CS) show characteristic low signal intensity stripes (black arrows) corresponding to the corticospinal tracts in a bulky basis pontis. The white arrow in f and g indicates the hypointense medial lemniscus (both images reprinted with permission from ref. 129). Axial T2-weighted image (h) shows characteristic symmetric hyperintensity of the peridentate cerebellar WM in a patient with cerebroendineous xanthomatosis (image reprinted with permission from [130]). Axial T2-weighted image (i) in a patient with Fragile X-associated tremor ataxia syndrome (FXTAS) shows symmetric hyperintensity of the cerebellar WM which is accompanied in a coronal T2-weighted FLAIR image (l) by symmetric extensive areas of hyperintensity of the cerebral WM and of the splenium of the corpus callosum (arrow). Joubert Joubert syndrome and related disorders; FRDA Friedreich's ataxia; AT ataxia telangectasia; ARSACS autosomal recessive spastic ataxia of Charlevoix-Saguenay; CTX cerebrotendineous xanthomatosis; FXTAS Fragile X-associated tremor ataxia syndrome
Similar articles
- Neuroimaging Biomarkers in SCA2 Gene Carriers.
Mascalchi M, Vella A. Mascalchi M, et al. Int J Mol Sci. 2020 Feb 4;21(3):1020. doi: 10.3390/ijms21031020. Int J Mol Sci. 2020. PMID: 32033120 Free PMC article. Review. - Magnetic resonance and nuclear medicine imaging in ataxias.
Mascalchi M, Vella A. Mascalchi M, et al. Handb Clin Neurol. 2012;103:85-110. doi: 10.1016/B978-0-444-51892-7.00004-8. Handb Clin Neurol. 2012. PMID: 21827882 Review. - Neuroimaging Applications in Chronic Ataxias.
Mascalchi M, Vella A. Mascalchi M, et al. Int Rev Neurobiol. 2018;143:109-162. doi: 10.1016/bs.irn.2018.09.011. Epub 2018 Oct 29. Int Rev Neurobiol. 2018. PMID: 30473193 Review. - Patterns of fractional anisotropy changes in white matter of cerebellar peduncles distinguish spinocerebellar ataxia-1 from multiple system atrophy and other ataxia syndromes.
Prakash N, Hageman N, Hua X, Toga AW, Perlman SL, Salamon N. Prakash N, et al. Neuroimage. 2009 Aug;47 Suppl 2:T72-81. doi: 10.1016/j.neuroimage.2009.05.013. Epub 2009 May 14. Neuroimage. 2009. PMID: 19446636 - Cerebellar disorders: clinical/radiologic findings and modern imaging tools.
Manto M, Habas C. Manto M, et al. Handb Clin Neurol. 2016;135:479-491. doi: 10.1016/B978-0-444-53485-9.00023-4. Handb Clin Neurol. 2016. PMID: 27432679 Review.
Cited by
- Neuroimaging Biomarkers in SCA2 Gene Carriers.
Mascalchi M, Vella A. Mascalchi M, et al. Int J Mol Sci. 2020 Feb 4;21(3):1020. doi: 10.3390/ijms21031020. Int J Mol Sci. 2020. PMID: 32033120 Free PMC article. Review. - Immune-Mediated Cerebellar Ataxias: Clinical Diagnosis and Treatment Based on Immunological and Physiological Mechanisms.
Mitoma H, Manto M, Hadjivassiliou M. Mitoma H, et al. J Mov Disord. 2021 Jan;14(1):10-28. doi: 10.14802/jmd.20040. Epub 2021 Jan 12. J Mov Disord. 2021. PMID: 33423437 Free PMC article. - Update on Paraneoplastic Cerebellar Degeneration.
Loehrer PA, Zieger L, Simon OJ. Loehrer PA, et al. Brain Sci. 2021 Oct 26;11(11):1414. doi: 10.3390/brainsci11111414. Brain Sci. 2021. PMID: 34827413 Free PMC article. Review. - Anti-MAG associated cerebellar ataxia and response to rituximab.
Zis P, Rao DG, Hoggard N, Sarrigiannis PG, Hadjivassiliou M. Zis P, et al. J Neurol. 2018 Jan;265(1):115-118. doi: 10.1007/s00415-017-8675-9. Epub 2017 Nov 20. J Neurol. 2018. PMID: 29159464 - Standardized Assessment of Hereditary Ataxia Patients in Clinical Studies.
Paap BK, Roeske S, Durr A, Schöls L, Ashizawa T, Boesch S, Bunn LM, Delatycki MB, Giunti P, Lehéricy S, Mariotti C, Melegh J, Pandolfo M, Tallaksen CME, Timmann D, Tsuji S, Schulz JB, van de Warrenburg BP, Klockgether T. Paap BK, et al. Mov Disord Clin Pract. 2016 Feb 11;3(3):230-240. doi: 10.1002/mdc3.12315. eCollection 2016 May-Jun. Mov Disord Clin Pract. 2016. PMID: 30363623 Free PMC article. Review.
References
- Mascalchi M, Vella A. Magnetic resonance and nuclear medicine imaging in ataxias. Handb Clin Neurol [Rev] 2012;103:85–110. - PubMed
- Currie S, Hadjivassiliou M, Craven IJ, Wilkinson ID, Griffiths PD, Hoggard N. Magnetic resonance imaging biomarkers in patients with progressive ataxia: current status and future direction. Cerebellum. 2013;12(2)::245–66. - PubMed
- Dohlinger S, Hauser TK, Borkert J, Luft AR, Schulz JB. Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum [Res Support Non-US Gov't] 2008;7(2):204–14. - PubMed
- Luft AR, Skalej M, Welte D, Kolb R, Burk K, Schulz JB, et al. A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Magn Reson Med [Clin Trial] 1998;40(1)::143–51. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical