Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens - PubMed (original) (raw)
. 2014 Nov 27;515(7528):577-81.
doi: 10.1038/nature13988.
Xiuli Zhang 2, Heiko Schuster 3, Etienne Caron 4, Jeffrey P Ward 5, Takuro Noguchi 1, Yulia Ivanova 1, Jasreet Hundal 6, Cora D Arthur 1, Willem-Jan Krebber 7, Gwenn E Mulder 7, Mireille Toebes 8, Matthew D Vesely 1, Samuel S K Lam 1, Alan J Korman 9, James P Allison 10, Gordon J Freeman 11, Arlene H Sharpe 12, Erika L Pearce 1, Ton N Schumacher 8, Ruedi Aebersold 13, Hans-Georg Rammensee 3, Cornelis J M Melief 14, Elaine R Mardis 15, William E Gillanders 2, Maxim N Artyomov 1, Robert D Schreiber 1
Affiliations
- PMID: 25428507
- PMCID: PMC4279952
- DOI: 10.1038/nature13988
Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens
Matthew M Gubin et al. Nature. 2014.
Abstract
The immune system influences the fate of developing cancers by not only functioning as a tumour promoter that facilitates cellular transformation, promotes tumour growth and sculpts tumour cell immunogenicity, but also as an extrinsic tumour suppressor that either destroys developing tumours or restrains their expansion. Yet, clinically apparent cancers still arise in immunocompetent individuals in part as a consequence of cancer-induced immunosuppression. In many individuals, immunosuppression is mediated by cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and programmed death-1 (PD-1), two immunomodulatory receptors expressed on T cells. Monoclonal-antibody-based therapies targeting CTLA-4 and/or PD-1 (checkpoint blockade) have yielded significant clinical benefits-including durable responses--to patients with different malignancies. However, little is known about the identity of the tumour antigens that function as the targets of T cells activated by checkpoint blockade immunotherapy and whether these antigens can be used to generate vaccines that are highly tumour-specific. Here we use genomics and bioinformatics approaches to identify tumour-specific mutant proteins as a major class of T-cell rejection antigens following anti-PD-1 and/or anti-CTLA-4 therapy of mice bearing progressively growing sarcomas, and we show that therapeutic synthetic long-peptide vaccines incorporating these mutant epitopes induce tumour rejection comparably to checkpoint blockade immunotherapy. Although mutant tumour-antigen-specific T cells are present in progressively growing tumours, they are reactivated following treatment with anti-PD-1 and/or anti-CTLA-4 and display some overlapping but mostly treatment-specific transcriptional profiles, rendering them capable of mediating tumour rejection. These results reveal that tumour-specific mutant antigens are not only important targets of checkpoint blockade therapy, but they can also be used to develop personalized cancer-specific vaccines and to probe the mechanistic underpinnings of different checkpoint blockade treatments.
Figures
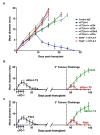
Extended Data Figure 1. Innate and adaptive immune components are required for rejection of d42m1-T3 after checkpoint blockade therapy
a, Cohorts of Rag2−/−, Batf3−/−, or wild type mice were treated with control mAb, αCD4, αCD8α, or αIFN-γ mAbs and then were injected with 1 x 106 d42m1-T3 tumour cells s.c. and subsequently treated with αCTLA-4 on days 3, 6, and 9 post-transplant. b, c, d42m1-T3 (b) or F244 (c) tumour cells were injected s.c. into WT mice (n=5) that were subsequently treated with αPD-1 on days 3, 6, and 9. Fifty days after tumours were rejected, mice were rechallenged with d42m1-T3 or F244 tumour cells. Data are presented as average tumour diameter ± s.e.m. of 5 mice per group and are representative of at least two independent experiments.
Extended Data Figure 2. H-2Db mutant epitopes of d42m1-T3 tumours
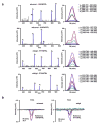
a, Missense mutations in d42m1-T3 were subjected to in silico analysis for the potential to form H-2Db binding epitopes using three epitope prediction algorithms. The median predicted epitope binding affinity for each peptide was calculated and expressed as “median affinity value” where affinity value = 1/IC50 x 100. Predicted epitopes are arrayed along the X-axis in alphabetical order based on their protein of origin b, Unfiltered median affinity values for the 4 predicted H-2Db epitopes. c, Median affinity values of remaining 2 H-2Db epitopes after filtering. d, Tetramer staining of CD8+ TILs from tumour bearing mice treated with αPD-1 using H-2Db tetramers loaded with top 4 H-2Db synthetic peptides. e, IFN-γ and TNF-α intracellular cytokine staining of CD8+ TILs from tumour bearing mice treated with αPD-1 immunotherapy following co-culture with naïve irradiated splenocytes pulsed with the top 4 H-2Db synthetic peptides added at 1 μM final concentration. Data are presented as per cent of CD8+ TILs positive for IFN-γ, TNF-α, or both cytokines. Data are representative of two independent experiments.
Extended Data Figure 3. mLama4 and mAlg8 stimulate CD8+ T cell lines generated against d42m1-T3 following αPD-1
a, CD8+ T cell lines generated from splenocytes of individual d42m1-T3 tumour bearing mice that rejected their tumours after αPD-1 therapy were incubated with irradiated d42m1-T3 tumour cells (or F244 tumour cells) treated with blocking mAb specific for H-2Kb, and/or H-2Db and IFN-γ production quantitated. Data are presented as means ± s.e.m and are representative of two independent experiments. Samples were compared using an unpaired, two-tailed Student’s t test (***p<0.001). b, IFN-γ release by the CTL 74 T cell line following co-culture with naïve irradiated splenocytes pulsed with the top 62 H-2Kb synthetic peptides added at 1 μM final concentration.
Extended Data Figure 4. mLama4 and mAlg8 bind H-2Kb and stimulate CD8+ T cell lines generated against d42m1-T3 following αPD-1 immunotherapy
a, IFN-γ release by CTL 62, CTL 73, or CTL 74 T cell lines following stimulation with naïve irradiated splenocytes pulsed with WT or mutant forms of Lama4 or Alg8 peptides. b, RMA-S cells were incubated with 8 amino acid peptides of mLama4 or mAlg8 and surface expression of H-2Kb or H-2Db was assessed by flow cytometry. Mean fluorescent intensity of H-2Kb and H-2Db was expressed as peptide binding score. Data presented are representative of at least two independent experiments.
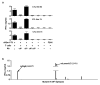
Extended Data Figure 5. Identification of a peptide bound to H-2Kb on d42m1-T3 tumour cells corresponding to mutant Lama4
a, Identification of the peptide, VGFNFRTL, corresponding to mLama4 by discovery MS. b, Validation of the mLama4 peptide using an isotope-labelled synthetic peptide (VGFNFRTL (13C6, 15N1)).
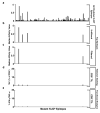
Extended Data Figure 6. Generation of SRM assay library for the detection of mutant H-2Kb peptides on d42m1-T3
a, SRM transitions were optimized for 51 of the 62 top predicted H-2Kb peptides. The 51 peptides chosen were selected based on having physiochemical properties that would allow their detection by MS if present. Only Lama4 and Alg8 are shown here for simplicity. The 51 peptides were synthesized and LC-MS/MS acquisition was performed on each peptide to determine the best collision energy and to obtain the full fragment ion spectrum (left panel); three to seven of the highest intensity peaks were selected to be built into SRM transitions. Optimal SRM transitions displayed as extracted ion chromatograms are shown (right panel). Q1 – Q3 transitions are indicated in parenthesis. The mutated amino acid in the peptide sequence is marked in red. b, F244 tumour cells, which lack the mLama4 and mAlg8 d42m1-T3 epitope, lack detectable mLama4 or mAlg8 in complex with H-2Kb as assessed by SRM.
Extended Data Figure 7. CD8+ T cells specific for mutant forms of Lama4 and Alg8 infiltrate d42m1-T3, but not F244, tumours
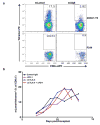
a, Detection of tumour infiltrating mLama4- or mAlg8-specific T cells infiltrating d42m1-T3 or F244 tumours of mice treated with αPD-1. Tumours were harvested on day 12 post-transplant. Cells were gated on live CD45+ and CD8α+ tumour infiltrating lymphocytes. Detection of mLama4- or mAlg8-specific T cells was achieved by staining with peptide-MHC H-2Kb PE-labelled tetramers. Data are representative of at least five independent experiments. b, Detection of mLama4-specific tumour infiltrating T cells from tumour-bearing mice treated with αPD-1, αCTLA-4, both αPD-1 plus αCTLA-4 or control mAb. Detection of mLama4-specific T cells was achieved by staining with mLama4-MHC H-2Kb PE-labelled tetramers. Data presented are plotted as the mean mLama4 tetramer-positive as a percent of CD8α+ tumour infiltrating cells and are representative of at least three independent experiments.
Extended Data Figure 8. mAlg8 and mLama4 SLP vaccine control d42m1-T3 tumour outgrowth when administered therapeutically or prophylactically
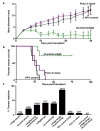
a, Tumour growth of d42m1-T3 tumours from mice therapeutically vaccinated with mLama4 and mAlg8 SLP plus Poly I:C, HPV control SLP plus Poly I:C or Poly I:C alone. Data shown are mean ± s.e.m. Mutant Lama4 and mAlg8 SLP vaccine group was compared to HPV control SLP vaccine group using an unpaired, two-tailed Student’s t test (*p<0.05 and **p<0.01) b, Kaplan-Meier survival curves of d42m1-T3 tumour bearing mice (7 per group) prophylactically vaccinated with SLP vaccines plus poly I:C. mLama4 plus mAlg8 compared to HPV control: p=0.0003 [log-rank (Mantel-Cox) test]. Representative of two independent experiments. c, Cumulative number of mice (7–10 per group) from at least two independent experiments rejecting d42m1-T3 or F244 tumours as a consequence of SLP or minimal epitope peptide prophylactic vaccination.
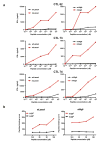
Extended Data Figure 9. Detection of TIM-3, LAG-3, IFN-γ and TNF-α expression in tumour infiltrating CD8+ T cells
a, Representative histogram of TIM-3 or LAG-3 expression on mLama4-specific CD8+ tumour infiltrating T cells from tumour bearing mice treated with αPD-1, αCTLA-4, both αPD-1 and αCTLA-4 or control mAbs. b, TIM-3 and LAG-3 are reduced in mAlg8-specific CD8+ TILs from tumour-bearing mice treated with αPD-1, αCTLA-4, or both αPD-1 and αCTLA-4 compared to mice treated with control mAb. N=5 mice per group pooled. Data are presented as mean ± s.e.m. of at least three independent experiments. Samples were compared using an unpaired, two-tailed Student’s t test (*p<0.05, **p<0.01). c, Representative dot plots of IFN-γ and TNF-α stained CD8+ tumour-infiltrating T cells from tumour-bearing mice following treatment with αPD-1, αCTLA-4, both αPD-1 and αCTLA-4 or control mAbs. Data presented are representative of at least three independent experiments.
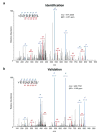
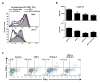
Figure 1. Mutations in Lama4 and Alg8 form top predicted d42m1-T3 epitopes
a, Growth of d42m1-T3 or F244 tumours in 5-mouse cohorts treated with αPD-1 (closed circles), αCTLA-4 (open circles), αPD-1+αCTLA-4 (open triangle) or control mAb (closed triangle). b, Potential H-2Kb binding epitopes predicted by in silico analysis of all missense mutations in d42m1-T3. c, Median affinity values for the top 62 predicted H-2Kb epitopes. d, Median affinity values of H-2Kb epitopes after filtering. e, Screening for specificities of CD8+ TILs from αPD-1 treated, d42m1-T3 tumour bearing mice using H-2Kb tetramers loaded with top 62 H-2Kb epitopes. f, IFN-γ and TNF-α induction in CD8+ TILs from αPD-1 treated, d42m1-T3 tumour bearing mice following culture with irradiated splenocytes pulsed with the top 62 H-2Kb peptides. Data are presented as per cent CD8+ TILs expressing IFN-γ, TNF-α or for both. Data are representative of two independent experiments.
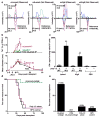
Figure 2. Mutant Lama4 and mAlg8 are therapeutically relevant d42m1-T3 TSMA
a, Detection of mLama4 and mAlg8 bound to cellular H-2Kb by mass spectrometry. b, Time dependent tumour infiltration of mLama4- and mAlg8-specific CD8+ T cells (n=5), (top). Data represent means ± s.e.m of 5 independent experiments. Growth kinetics of d42m1-T3 and F244 during αPD-1 immunotherapy (n=5), (bottom). Data represent average tumour diameter ± s.e.m. and are representative of at least three independent experiments. c, IFN-γ ELISPOT analysis of peptide stimulated splenocytes from mice immunized with mLama4 or mAlg8 SLP plus polyI:C (n=3 mice per group). Data are means ± s.e.m. Representative of two independent experiments. Samples were compared using unpaired, two-tailed Student’s t test (*p<0.05, **p<0.01). d, Kaplan-Meier survival curves of d42m1-T3 tumour bearing mice (10 mice per group) therapeutically vaccinated with SLP vaccines plus poly I:C. mLama4 plus mAlg8 compared to HPV control: p=0.0002 [log-rank (Mantel-Cox) test]. Representative of two independent experiments. e, Cumulative data from two independent SLP therapeutic vaccine experiments using mice (7–10 per group) with d42m1-T3 or F244 tumours.
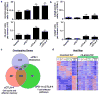
Figure 3. Differential effects of checkpoint blockade therapy on tumour antigen-specific CD8+ T cells
a, b, Per cent of CD8+ TILs specific for mLama4 (a) or mAlg8 (b) following checkpoint blockade therapy (top). Mean number of mLama4- (a) or mAlg8- (b) specific CD8+ TILs per tumour following checkpoint blockade therapy (bottom). N=5 mice per group pooled. Data are means ± s.e.m. of at least three independent experiments. Samples were compared to control mAb treatment using unpaired, two-tailed Student’s t test (*p<0.05, **p<0.01). c, Venn diagram revealing relationships between differentially expressed genes (p<0.05) in mLama4-specific CD8+ TILs from mice treated with checkpoint blocking mAbs versus control mAb. d, Heatmap showing differentially expressed genes (p<0.05) in mLama4-specific CD8+ TILs from mice treated with checkpoint blocking versus control mAbs. Colour pattern is relative with respect to the row, with red indicating gene upregulation and blue indicating gene downregulation.
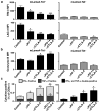
Figure 4. Checkpoint blockade therapy alters the functional phenotypes of tumour antigen-specific CD8+ T cells
a, TIM-3 or LAG-3 expression on CD8+ TILs following checkpoint blockade therapy. b, Granzyme B expression in CD8+ TILs following checkpoint blockade therapy. c, Per cent of CD8+ TILs positive for IFN-γ and/or TNF-α following checkpoint blockade therapy. N=5 mice per group pooled. Data are means ± s.e.m. of at least three independent experiments. Samples were compared to control mAb treated mice using an unpaired, two-tailed Student’s t test (*p<0.05, **p<0.01).
Comment in
- Immunotherapy: personalizing tumour vaccines.
Killock D. Killock D. Nat Rev Clin Oncol. 2015 Feb;12(2):64. doi: 10.1038/nrclinonc.2014.220. Epub 2014 Dec 16. Nat Rev Clin Oncol. 2015. PMID: 25511189 No abstract available.
Similar articles
- Immunotherapy: personalizing tumour vaccines.
Killock D. Killock D. Nat Rev Clin Oncol. 2015 Feb;12(2):64. doi: 10.1038/nrclinonc.2014.220. Epub 2014 Dec 16. Nat Rev Clin Oncol. 2015. PMID: 25511189 No abstract available. - Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8+ T cells.
Fehlings M, Simoni Y, Penny HL, Becht E, Loh CY, Gubin MM, Ward JP, Wong SC, Schreiber RD, Newell EW. Fehlings M, et al. Nat Commun. 2017 Sep 15;8(1):562. doi: 10.1038/s41467-017-00627-z. Nat Commun. 2017. PMID: 28916749 Free PMC article. - CD27 Agonism Plus PD-1 Blockade Recapitulates CD4+ T-cell Help in Therapeutic Anticancer Vaccination.
Ahrends T, Bąbała N, Xiao Y, Yagita H, van Eenennaam H, Borst J. Ahrends T, et al. Cancer Res. 2016 May 15;76(10):2921-31. doi: 10.1158/0008-5472.CAN-15-3130. Epub 2016 Mar 28. Cancer Res. 2016. PMID: 27020860 - The present status and future prospects of peptide-based cancer vaccines.
Hirayama M, Nishimura Y. Hirayama M, et al. Int Immunol. 2016 Jul;28(7):319-28. doi: 10.1093/intimm/dxw027. Epub 2016 May 28. Int Immunol. 2016. PMID: 27235694 Review. - Immune Checkpoint Blockade in Breast Cancer Therapy.
Bu X, Yao Y, Li X. Bu X, et al. Adv Exp Med Biol. 2017;1026:383-402. doi: 10.1007/978-981-10-6020-5_18. Adv Exp Med Biol. 2017. PMID: 29282694 Review.
Cited by
- Neoantigen DNA vaccines are safe, feasible, and induce neoantigen-specific immune responses in triple-negative breast cancer patients.
Zhang X, Goedegebuure SP, Chen MY, Mishra R, Zhang F, Yu YY, Singhal K, Li L, Gao F, Myers NB, Vickery T, Hundal J, McLellan MD, Sturmoski MA, Kim SW, Chen I, Davidson JT 4th, Sankpal NV, Myles S, Suresh R, Ma CX, Foluso A, Wang-Gillam A, Davies S, Hagemann IS, Mardis ER, Griffith O, Griffith M, Miller CA, Hansen TH, Fleming TP, Schreiber RD, Gillanders WE. Zhang X, et al. Genome Med. 2024 Nov 14;16(1):131. doi: 10.1186/s13073-024-01388-3. Genome Med. 2024. PMID: 39538331 Free PMC article. Clinical Trial. - Neoantigen architectures define immunogenicity and drive immune evasion of tumors with heterogenous neoantigen expression.
Roerden M, Castro AB, Cui Y, Harake N, Kim B, Dye J, Maiorino L, White FM, Irvine DJ, Litchfield K, Spranger S. Roerden M, et al. J Immunother Cancer. 2024 Nov 9;12(11):e010249. doi: 10.1136/jitc-2024-010249. J Immunother Cancer. 2024. PMID: 39521615 Free PMC article. - Probiotic neoantigen delivery vectors for precision cancer immunotherapy.
Redenti A, Im J, Redenti B, Li F, Rouanne M, Sheng Z, Sun W, Gurbatri CR, Huang S, Komaranchath M, Jang Y, Hahn J, Ballister ER, Vincent RL, Vardoshivilli A, Danino T, Arpaia N. Redenti A, et al. Nature. 2024 Nov;635(8038):453-461. doi: 10.1038/s41586-024-08033-4. Epub 2024 Oct 16. Nature. 2024. PMID: 39415001 Free PMC article. - Short Peptides as Powerful Arsenal for Smart Fighting Cancer.
Bojarska J, Wolf WM. Bojarska J, et al. Cancers (Basel). 2024 Sep 24;16(19):3254. doi: 10.3390/cancers16193254. Cancers (Basel). 2024. PMID: 39409876 Free PMC article. Review. - Losartan rewires the tumor-immune microenvironment and suppresses IGF-1 to overcome resistance to chemo-immunotherapy in ovarian cancer.
Sun Y, Yin Z, Li S, Wu L, Zhang Y, Zhao Y, Gomes Dos Santos IL, Subudhi S, Lei P, Muzikansky A, Yuan Z, Rueda BR, Jain RK, Xu L. Sun Y, et al. Br J Cancer. 2024 Nov;131(10):1683-1693. doi: 10.1038/s41416-024-02863-9. Epub 2024 Oct 5. Br J Cancer. 2024. PMID: 39369055
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA190700/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U01 CA141541/CA/NCI NIH HHS/United States
- P50 CA101942/CA/NCI NIH HHS/United States
- R37 CA043059/CA/NCI NIH HHS/United States
- T32 CA009547/CA/NCI NIH HHS/United States
- P30 AR048335/AR/NIAMS NIH HHS/United States
- P01 AI054456/AI/NIAID NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 AI091965/AI/NIAID NIH HHS/United States
- R01 CA043059/CA/NCI NIH HHS/United States
- T32 CA00954729/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases