Metalloproteinase-dependent TLR2 ectodomain shedding is involved in soluble toll-like receptor 2 (sTLR2) production - PubMed (original) (raw)
Metalloproteinase-dependent TLR2 ectodomain shedding is involved in soluble toll-like receptor 2 (sTLR2) production
Patricia Langjahr et al. PLoS One. 2014.
Abstract
Toll-like receptor (TLR) 2, a type I membrane receptor that plays a key role in innate immunity, recognizes conserved molecules in pathogens, and triggering an inflammatory response. It has been associated with inflammatory and autoimmune diseases. Soluble TLR2 (sTLR2) variants have been identified in human body fluids, and the TLR2 ectodomain can negatively regulate TLR2 activation by behaving as a decoy receptor. sTLR2 generation does not involve alternative splicing mechanisms, indicating that this process might involve a post-translational modification of the full-length receptor; however, the specific mechanism has not been studied. Using CD14+ peripheral human monocytes and the THP-1 monocytic leukemia-derived cell line, we confirm that sTLR2 generation increases upon treatment with pro-inflammatory agents and requires a post-translational mechanism. We also find that the constitutive and ligand-induced release of sTLR2 is sensitive to pharmacological metalloproteinase activator and inhibitors leading us to conclude that metalloproteinase TLR2 shedding contributes to soluble receptor production. By expressing human TLR2 in ADAM10- or ADAM17-deficient MEF cells, we find both enzymes to be implicated in TLR2 ectodomain shedding. Moreover, using a deletion mutant of the TLR2 juxtamembrane region, we demonstrate that this domain is required for sTLR2 generation. Functional analysis suggests that sTLR2 generated by metalloproteinase activation inhibitsTLR2-induced cytokine production by this monocytic leukemia-derived cell line. The identification of the mechanisms involved in regulating the availability of soluble TLR2 ectodomain and cell surface receptors may contribute further research on TLR2-mediated processes in innate immunity and inflammatory disorders.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
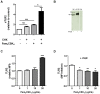
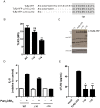
Figure 1. Production of sTLR2 involves a post-translation mechanism.
(A) THP-1 cells were pretreated or not with cycloheximide (100 µg/mL) for 30 min and stimulated with Pam3CSK4 (1 µg/mL) for 5 h and the amount of sTLR2 was quantified in the cell culture supernatants by ELISA. Student t test, * p<0.05. (B) Detection of sTLR2 in 18 h culture supernatant (SN) of THP-1 cells by Western blotting using a N-terminal anti-TLR2 antibody. One representative of three experiments is shown. SN: cell culture supernatant; rhTLR2: recombinant human TLR2 protein. (C) Cell surface TLR2 levels evaluated by flow cytometry after stimulation with Pam3CSK4 (1, 10 or 20 µg/mL) for 2 h. ***, p<0.0001. (D) Cell surface TLR2 levels evaluated by flow cytometry after treatment with cycloheximide and then stimulation for 2 h with Pam3CSK4. MFI = mean fluorescence intensity. *, p<0.05; **, p = 0.009.
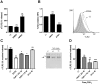
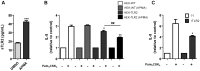
Figure 2. Metalloproteinase activity regulates sTLR2 release and TLR2 surface content from THP-1 cells.
(A) Cells were treated with APMA (10 µM), vehicle (DMSO) or left untreated (-) for 5 h and the concentration of sTLR2 in the supernatants was determined by ELISA. Student t-test, **, p = 0.003. (B) Cell surface TLR2 was examined on these cells after 2 h addition of APMA (10 µM), vehicle (DMSO) or left untreated (-) using anti-TLR2-FITC conjugated antibody and analysis by flow cytometry. ***, p<0.0001. MFI = mean fluorescence intensity. A representative histogram is shown using cells stained with isotype control antibody (filled histogram), untreated cells (dot line) and APMA-treated cells (black line). (C) Cells were treated for 18 h with EDTA (2 mM), TAPI-1 (25 µM or 75 µM), GI254023X (5 µM) (GI) or left untreated (-) and the sTLR2 concentration in the cell supernatants was determined by ELISA. *, p<0.05; **, p = 0.003. Westernblot (10% SDS-PAGE reducing gel) shows sTLR2 release in cell supernatant of TAPI-1 or left untreated (-) treated THP-1 cells using N-terminal anti-TLR2 antibody. One representative of three experiments is shown. (D) Cells treated with TAPI-1 (25 µM or 75 µM), GI254023X (GI) (5 µM) or left untreated (-) were added 30 min prior to cell stimulation with Pam3CSK4 (1 µg/mL) for 18 h and the concentration of sTLR2 in cell supernatants was assayed by ELISA. Data represent the mean ± SE of three independent determinations. Student t test **, p = 0.001; ***, p = 0.0001.
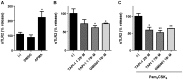
Figure 3. TLR2 shedding is involved in sTLR2 generation in human peripheral monocytes.
(A) Isolated human peripheral CD14+ cells were treated with APMA (10 µM), vehicle (DMSO) or left untreated (-) for 5 h. *, p<0.05. (B) Cells were incubated for 18 h with TAPI-1 (25 µM or 75 µM), GM6001 (10 µM) or left untreated (-).*, p<0.05.(C) Cells treated with TAPI-1 (25 µM or 75 µM), GI254023X (GI) (5 µM) or left untreated (-) were added 30 min prior to cell stimulation with Pam3CSK4 (1 µg/mL) for 18 h. Supernatants were harvested and soluble receptor was measured by ELISA. *, p<0.05; ** p<0.01. Data are express as percentage of maximal release ± SE of two independent determinations using four different healthy donors.
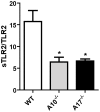
Figure 4. ADAM10 and ADAM17 are involved in TLR2 shedding.
MEF cells were transfected with an expression plasmid encoding human TLR2 or with an empty vector; sTLR2 content in cell supernatants was analyzed by ELISA. sTLR2 content (pg/mL) was normalized to total TLR2 cell levels (ng/mg total protein). Data represent the mean ± SE of three independent determinations. *, p<0.05.
Figure 5. Deletion of TLR2 juxtamembrane region impairs sTLR2 generation.
(A)Amino acid sequence of wild type (TLR2-YFP) surrounding the potential TLR2 transmembrane domain and deletion mutants, lacking 10 (TLR2-YFP-Δ10) or 16 (TLR2-YFP-Δ16) amino acids. Student t test, ** p<0.01. (B) HEK293T cells were transiently transfected with TLR2-YFP, TLR2-YFP-Δ10 or TLR2-YFP-Δ16, and TLR2 surface expression was evaluated by flow cytometry with anti-TLR2-PE antibody. MFI = mean fluorescence intensity. Student t test, ** p<0.01. (C) Westernblot analysis, using anti-GFP antibody, of transfected cell lysates described in (B) Mock transfected HEK-cells (-); bands correspond to two GFP immunoreactive TLR2-YFP polypeptides. (D) Pam3CSK4-induced IL-8 production by wild type and deletion mutant expressing cells was evaluated by ELISA. (E) sTLR2 content in cell supernatants, relative to Pam3CSK4-induced wild type cells, of wild type or Δ10 or Δ16 deletion mutant transfected cells was evaluated by ELISA. * p<0.05; ** p<0.01. All graphs show mean values ± SE of three independent experiments.
Figure 6. sTLR2 induced by metalloproteinase activator APMA inhibit TLR2 ligand-mediated IL-8 production.
(A) Stably transfected HEK-TLR2-YFP cells were incubated for 24 h with fresh medium; 5 h prior to cell supernatant collections they were treated with APMA (10 µM) or DMSO; and sTLR2 content in conditioned media was determined by ELISA. Student t test ***, p<0.0001. (B) THP-1 cells were pre-treated with conditioned media of HEK293 (HEK-WT), APMA-treated HEK293 (HEK-WT+APMA), HEK-TLR2-YFP (HEK-TLR2) or APMA-treated HEK-TLR2-YFP cells (HEK-TLR2+APMA), and then Pam3CSK4-induced IL-8 production was determined. Student t test *, p = 0.0481; **, p = 0.0017; ##, p = 0.008. (C) Pam3CSK4-induced IL-8 production by THP-1 cells, pre-treated or not with recombinant human TLR2 ectodomain. Student t test *, p<0.05. All graphs show mean values ± SE of three independent experiments.
Similar articles
- Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk.
LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP, Labéta MO. LeBouder E, et al. J Immunol. 2003 Dec 15;171(12):6680-9. doi: 10.4049/jimmunol.171.12.6680. J Immunol. 2003. PMID: 14662871 - Human parotid saliva contains soluble toll-like receptor (TLR) 2 and modulates TLR2-mediated interleukin-8 production by monocytic cells.
Kuroishi T, Tanaka Y, Sakai A, Sugawara Y, Komine K, Sugawara S. Kuroishi T, et al. Mol Immunol. 2007 Mar;44(8):1969-76. doi: 10.1016/j.molimm.2006.09.028. Epub 2006 Nov 1. Mol Immunol. 2007. PMID: 17081611 - Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection.
Dulay AT, Buhimschi CS, Zhao G, Oliver EA, Mbele A, Jing S, Buhimschi IA. Dulay AT, et al. J Immunol. 2009 Jun 1;182(11):7244-53. doi: 10.4049/jimmunol.0803517. J Immunol. 2009. PMID: 19454721 - Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments.
Lichtenthaler SF, Lemberg MK, Fluhrer R. Lichtenthaler SF, et al. EMBO J. 2018 Aug 1;37(15):e99456. doi: 10.15252/embj.201899456. Epub 2018 Jul 5. EMBO J. 2018. PMID: 29976761 Free PMC article. Review. - Meprin and ADAM proteases as triggers of systemic inflammation in sepsis.
Rahn S, Becker-Pauly C. Rahn S, et al. FEBS Lett. 2022 Mar;596(5):534-556. doi: 10.1002/1873-3468.14225. Epub 2021 Nov 18. FEBS Lett. 2022. PMID: 34762736 Review.
Cited by
- The Presence of Psoriasis, Metabolic Syndrome and Their Combination Increases the Serum Levels of CRP and CD5L but Not sCD200R1 and sTLR2 in Participants.
Holmannova D, Borsky P, Andrys C, Krejsek J, Cermakova E, Fiala Z, Hamakova K, Svadlakova T, Parova H, Rehacek V, Poctova G, Borska L. Holmannova D, et al. J Pers Med. 2022 Nov 28;12(12):1965. doi: 10.3390/jpm12121965. J Pers Med. 2022. PMID: 36556186 Free PMC article. - The Crucial Triad: Endothelial Glycocalyx, Oxidative Stress, and Inflammation in Cardiac Surgery-Exploring the Molecular Connections.
Ćurko-Cofek B, Jenko M, Taleska Stupica G, Batičić L, Krsek A, Batinac T, Ljubačev A, Zdravković M, Knežević D, Šoštarič M, Sotošek V. Ćurko-Cofek B, et al. Int J Mol Sci. 2024 Oct 10;25(20):10891. doi: 10.3390/ijms252010891. Int J Mol Sci. 2024. PMID: 39456673 Free PMC article. Review. - Immune Responses to IAV Infection and the Roles of L-Selectin and ADAM17 in Lymphocyte Homing.
Reed SG, Ager A. Reed SG, et al. Pathogens. 2022 Jan 25;11(2):150. doi: 10.3390/pathogens11020150. Pathogens. 2022. PMID: 35215094 Free PMC article. Review. - Acute high-intensity interval exercise reduces human monocyte Toll-like receptor 2 expression in type 2 diabetes.
Durrer C, Francois M, Neudorf H, Little JP. Durrer C, et al. Am J Physiol Regul Integr Comp Physiol. 2017 Apr 1;312(4):R529-R538. doi: 10.1152/ajpregu.00348.2016. Epub 2017 Jan 25. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28122717 Free PMC article. Clinical Trial. - Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation.
He Y, Lawlor NT, Newburg DS. He Y, et al. Adv Nutr. 2016 Jan 15;7(1):102-11. doi: 10.3945/an.115.010090. Print 2016 Jan. Adv Nutr. 2016. PMID: 26773018 Free PMC article. Review.
References
- Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216. - PubMed
- Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. - PubMed
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820. - PubMed
- Coll RC, O'Neill LA (2010) New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J Innate Immun 2:406–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous