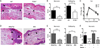Dermal-resident versus recruited γδ T cell response to cutaneous vaccinia virus infection - PubMed (original) (raw)
Dermal-resident versus recruited γδ T cell response to cutaneous vaccinia virus infection
Amanda S Woodward Davis et al. J Immunol. 2015.
Abstract
The study of T cell immunity at barrier surfaces has largely focused on T cells bearing the αβ TCR. However, T cells that express the γδ TCR are disproportionately represented in peripheral tissues of mice and humans, suggesting they too may play an important role responding to external stimuli. In this article, we report that, in a murine model of cutaneous infection with vaccinia virus, dermal γδ T cell numbers increased 10-fold in the infected ear and resulted in a novel γδ T cell population not found in naive skin. Circulating γδ T cells were specifically recruited to the site of inflammation and differentially contributed to dermal populations based on their CD27 expression. Recruited γδ T cells, the majority of which were CD27(+), were granzyme B(+) and made up about half of the dermal population at the peak of the response. In contrast, recruited and resident γδ T cell populations that made IL-17 were CD27(-). Using a double-chimera model that can discriminate between the resident dermal and recruited γδ T cell populations, we demonstrated their divergent functions and contributions to early stages of tissue inflammation. Specifically, the loss of the perinatal thymus-derived resident dermal population resulted in decreased cellularity and collateral damage in the tissue during viral infection. These findings have important implications for our understanding of immune coordination at barrier surfaces and the contribution of innate-like lymphocytes on the front lines of immune defense.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures
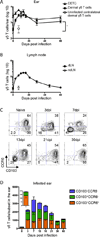
Figure 1
Following skin scarification with Vaccinia virus (VV), γδ T cells accumulate in draining lymph node and dermis. TCRδ-GFP mice were infected with VV on the ear by scarification. γδ T cells were gated as TCRβ−GFP+ in the LN and as TCRβ−GFP+Vγ5+ (Dendritic Epidermal T Cells, DETC) or TCRβ−GFP+Vγ5− (dermal γδ T cells) in the ear. A) Number of DETC and dermal γδ T cells in the ear following infection. Open symbol represents the number of dermal γδ T cells in the contralateral uninfected ear at 7dpi. B) Number of γδ T cells in the dLN following infection. Open symbol represents the number of γδ T cells in the contralateral non-draining LN (ndLN) at 7dpi. C) Representative flow plots show CCR6 and CD103 profile of dermal γδ T cells following infection. Numbers denote percent of total dermal γδ T cell population within the indicated quadrant. D) Cell numbers within the indicated dermal γδ T cell subsets over the course of infection per ear (insert, day 0). Error bars signify SEM. Data are compiled from >5 (A, B) or >3 (C, D) independent experiments, n=3–13 mice per group. Statistics performed with the two-tailed, unpaired _t_-test, *p<0.001.
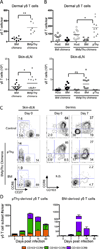
Figure 2
Circulating γδ T cells migrate specifically to VV infected skin and contribute to all three dermal subsets. C57BL/6 mice (CD45.2) or CD45.2+ TCRδ-GFP mice received 7×105 γδ T cells isolated from spleen and skin dLN of CD45.1+ TCRδ-GFP mice. The following day, mice were infected with VV on the ear. A) Graphs show numbers of transferred TCRδ-GFP+ cells recovered from the LN or ear, shown as +/− SEM. B) Representative flow plot shows the CCR6 and CD27 profile of the donor γδ T cell population prior to transfer. C) Representative flow plots show CCR6 and CD103 profile of donor and recipient dermal cells at 7dpi. D) γδ T cells from spleen and skin dLN of TCRδ-GFP mice were sorted based on CD27 expression and 6×105 CD27+ or 2.7×105 CD27− γδ T cells were transferred into separate C57BL/6 hosts. Recipients were infected with VV on the ear the following day. Flow plots show the phenotype of CD27− and CD27+ at the time of transfer and 7dpi in the spleen and ear. Numbers denote the percentage of cells within the indicated quadrant. Data are representative of at least two independent experiments, n=3–7 mice per group.
Figure 3
Contribution of adult BM-derived and perinatal thymus-derived γδ T cells to the dermal response. CD45.2+ TCRδ-GFP mice were irradiated and reconstituted with BM (BM only) or with BM plus pThy (BMpThy). A) Graphs show the total number of γδ T cells > 8 weeks following reconstitution in the dermis and ski dLN. Geometric mean is shown. B) Contribution of BM-derived and pThy-derived γδ T cells in the dermis and LN. Geometric mean is shown. C) and D) BMpThy chimeras were infected with VV on the ear > 8 weeks following reconstitution. C) CCR6 and CD27 profiles of γδ T cells in the skin dLN and CCR6 and CD103 profiles of γδ T cells in the dermis at day 0 and 7dpi (N.D. = not done). Numbers denote the percentage of that population within the indicated quadrant. D) The number of BM- and pThy-derived γδ T cells contributing to subsets in the dermis following infection. Error bars signify SEM. Statistics performed with the two-tailed, unpaired student _t_-test, *p<0.02, **p<0.0001, NS = not significant. Data show results from >3 (A, B) or >2 independent experiments (C, D), n=3–15 mice per group.
Figure 4
Adult BM-derived and pThy-derived γδ T cells in the dermis are functionally distinct. A) TCRδ-GFP mice and B) BMpThy chimeras were infected with VV on the ear and sacrificed on days 3 and 7. Ears were digested in Brefeldin A and cells were re-stimulated with PMA/Ionomycin for 4 hours at 37°C followed by intracellular staining for cytokines and Granzyme B. Representative flow plots are shown and gates are based on unstimulated naïve samples. Numbers indicate the percentage of that population in each quadrant. Data are representative of at >2 independent experiments.
Figure 5
Dermal γδ T cells accelerate early collateral damage and contribute to increased tissue cellularity during VV infection. Mice were infected with VV on the ear. A) Representative histological sections of ears from C57BL/6 WT and TCRδ−/− mice 3dpi at 20× magnification. B) Graphs show scores for necrosis (epidermis and cartilage scored separately and then combined) and cellularity. Mean +/− SEM is shown. C) WT and TCRδ−/−mice were sacrificed at the indicated time points following infection and ears were taken for plaque assay. Error bars indicate SEM. D) Representative histological sections and E) scores for BM chimeras and BMpThy chimeras 3dpi. Mean +/− SEM is shown. F) Comparison of necrosis scores for WT, TCRδ−/−, BMpThy chimeras and BM chimeras in relation to the presence (+) or absence (−) of γδ T cell populations (Circ = circulating γδ T cells, Derm = dermal γδ T cells). Arrows indicate necrotic cartilage, arrowheads indicate neutrophilic infiltrates and asterisks indicate serocellular crusts. Statistics performed with the two-tailed, unpaired student _t_-test, n=5–6 mice per group. *p<0.05, **p<0.01, ***p<0.005 Data are representative of >1 independent experiments, n=3–6 mice per group (C) or >2 (A, B, D, E) independent experiments, n=5–6 mice per group.
Similar articles
- Dermal γδ T Cells Do Not Freely Re-Circulate Out of Skin and Produce IL-17 to Promote Neutrophil Infiltration during Primary Contact Hypersensitivity.
Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS. Jiang X, et al. PLoS One. 2017 Jan 12;12(1):e0169397. doi: 10.1371/journal.pone.0169397. eCollection 2017. PLoS One. 2017. PMID: 28081153 Free PMC article. - Innate immunity to viruses: control of vaccinia virus infection by gamma delta T cells.
Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Selin LK, et al. J Immunol. 2001 Jun 1;166(11):6784-94. doi: 10.4049/jimmunol.166.11.6784. J Immunol. 2001. PMID: 11359837 - CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets.
Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. Ribot JC, et al. Nat Immunol. 2009 Apr;10(4):427-36. doi: 10.1038/ni.1717. Epub 2009 Mar 8. Nat Immunol. 2009. PMID: 19270712 Free PMC article. - Differentiation and activation of γδ T Lymphocytes: Focus on CD27 and CD28 costimulatory receptors.
Ribot JC, Silva-Santos B. Ribot JC, et al. Adv Exp Med Biol. 2013;785:95-105. doi: 10.1007/978-1-4614-6217-0_11. Adv Exp Med Biol. 2013. PMID: 23456842 Review. - [T gamma-delta lymphocytes and their role in hypersensitivity processes in the digestive and respiratory mucosa].
Villarrubia N, León F, Bootello A. Villarrubia N, et al. Allergol Immunopathol (Madr). 2002 Sep-Oct;30(5):273-82. Allergol Immunopathol (Madr). 2002. PMID: 12396962 Review. Spanish.
Cited by
- T Cell Surveillance during Cutaneous Viral Infections.
Pei L, Hickman HD. Pei L, et al. Viruses. 2024 Apr 26;16(5):679. doi: 10.3390/v16050679. Viruses. 2024. PMID: 38793562 Free PMC article. Review. - Murine CXCR3+CXCR6+γδT Cells Reside in the Liver and Provide Protection Against HBV Infection.
Wang Y, Guan Y, Hu Y, Li Y, Lu N, Zhang C. Wang Y, et al. Front Immunol. 2022 Jan 21;12:757379. doi: 10.3389/fimmu.2021.757379. eCollection 2021. Front Immunol. 2022. PMID: 35126348 Free PMC article. - The Roles of Liver-Resident Lymphocytes in Liver Diseases.
Wang Y, Zhang C. Wang Y, et al. Front Immunol. 2019 Jul 16;10:1582. doi: 10.3389/fimmu.2019.01582. eCollection 2019. Front Immunol. 2019. PMID: 31379818 Free PMC article. Review. - Immunological memories of the bone marrow.
Chang HD, Tokoyoda K, Radbruch A. Chang HD, et al. Immunol Rev. 2018 May;283(1):86-98. doi: 10.1111/imr.12656. Immunol Rev. 2018. PMID: 29664564 Free PMC article. Review. - IL-17A-producing resident memory γδ T cells orchestrate the innate immune response to secondary oral Listeria monocytogenes infection.
Romagnoli PA, Sheridan BS, Pham QM, Lefrançois L, Khanna KM. Romagnoli PA, et al. Proc Natl Acad Sci U S A. 2016 Jul 26;113(30):8502-7. doi: 10.1073/pnas.1600713113. Epub 2016 Jul 11. Proc Natl Acad Sci U S A. 2016. PMID: 27402748 Free PMC article.
References
- Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013;14:978–985. - PubMed
- Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 2013;3:238–245. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials