αVβ3 Integrin Regulation of Respiratory Burst in Fibrinogen Adherent Human Neutrophils - PubMed (original) (raw)
αVβ3 Integrin Regulation of Respiratory Burst in Fibrinogen Adherent Human Neutrophils
Hye-Yeong Kim et al. Cell Mol Bioeng. 2014 Jun.
Abstract
In response to inflammatory stimuli, microvascular endothelial cells become activated, initiating the capture and exit of neutrophils from the blood vessel and into the extravascular extracellular matrix (ECM). In the extravascular space, neutrophils bind to ECM proteins, regulating cellular functions via signaling through adhesion molecules known as integrins. The αVβ3 integrin is an important mediator of neutrophil adhesion to ECM proteins containing the Arg-Gly-Asp (RGD) peptide sequence, including fibrinogen and fibronectin. Despite the abundance of RGD sequence in the ECM, adhesion molecule-mediated neutrophil activity has been focused on the β2 (Mac-1, CD11b/CD18) and β1 integrin response to matrix proteins. Here we investigated αVβ3 integrin-mediated reactive oxidant suppression as a consequence of human neutrophil adhesion to RGD containing proteins. Using integrin ligand-modified (poly)ethylene glycol hydrogels and reactive oxygen species (ROS) sensitive fluorescent probes (dihydrotetramethylrhosamine, H2TMRos), we evaluated integrin-peptide interactions that effectively regulate ROS generation. This study demonstrates that neutrophil adhesion suppresses ROS production in an αVβ3-dependent manner. Additionally, we determine that p38 mitogen-activated protein kinase in the respiratory burst signaling pathway is interrupted by integrin-mediated adhesion. These data indicate that ECM/integrin interactions can induce αVβ3-mediated adhesion dependent downstream signaling of ROS regulation via a Mac-1 independent mechanism.
Keywords: Adhesion; Extracellular matrix (ECM); Polyethylene glycol (PEG); Reactive oxygen species (ROS).
Conflict of interest statement
Conflict of interest
Hye-Yeong Kim, Eleni A. Skokos, Deborah J. Myer, Perez Agaba, and Anjelica L. Gonzalez declare that they have no conflicts of interest.
Figures
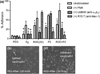
Figure 1
Neutrophil adhesion on PEG, fibrinogen (Fg), and integrin ligand modified hydrogels. (a) Cells were pre-incubated with PMA (100 nM), anti-αVβ3 integrin antibody (LM609, 10 μg/mL) or anti-β2 integrin antibody (R15.7, 10 μg/mL) and standard 500 s adhesion assays were performed. Values represent means taken from a minimum of three experiments with a minimum of three blood donors ± SE. #Difference compared to PEG (unstimulated cells), p < 0.001; *Difference compared to PEG (unstimulated cells), p < 0.01, +Difference when compared with PMA stimulated cells on peptide without antibody inhibition, p < 0.05. (b) Brightfield images of neutrophils settled on the surface of the PEG hydrogel after 120 min and (c) neutrophils firmly attached to RGD hydrogels after 120 min
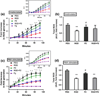
Figure 2
Integrin-mediated ROS suppression of neutrophils. Integrin-mediated ROS generations were evaluated by measuring fluorescent oxidation product of H2TMRos (λex/λem = 544/590 nm). ROS production from (a) unstimulated and (c) PMA-stimulated neutrophils on fibrinogen, PEG, RGD, P2, and RGD+P2 modified hydrogels. ±SE of >three independent experiments. *Difference between cells on RGD and PEG, p < 0.05, #Difference between cells on P2 and PEG, p < 0.05, ^Difference between cells on RGD+P2 and PEG, p < 0.05. (b) and (d) reflect total ROS suppression from the area AUC relative to the total ROS detected from neutrophils on PEG. Values represent the means of triplicates. **Difference when compared with cells on PEG, p < 0.01, #Difference when compared with cells on RGD, p < 0.05
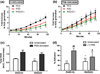
Figure 3
ROS production as an effect of RGD concentration. Time-course ROS monitoring from (a) untreated and (b) PMA-stimulated neutrophils seeded on hydrogels containing PEG alone, RGD and RGD(2X). *Difference between RGD(2X) and PEG, p < 0.01, %Difference between RGD(1X) and RGD(2X), p < 0.01. (c) Total ROS suppression from adherent neutrophils to the RGD using AUC. Values represent the means of triplicates ± SE of >three independent experiments. **Difference between RGD(1X) and RGD(2X), p < 0.01. (d) % adhesion of unstimulated and PMA-stimulated cells on RGD(1X) and RGD(2X). ***Difference between the PEG and RGD containing gels, p < 0.0001, *Difference between RGD(1X) and RGD(2X), p < 0.05, #Difference between unstimulated and PMA-stimulated cells, ###p < 0.0001, #p < 0.05
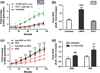
Figure 4
Effects of anti-integrin antibody blocking and free RGD blocking of adhesion on ROS secretion. Neutrophils were pre-incubated with 10 μg/mL of αVβ3 integrin antibody (LM609) or β2 integrin antibody (R15.7) and seeded on the gels. ROS production from PEG hydrogels containing (a) RGD. *Difference when compared to untreated neutrophils on RGD p < 0.05; (b) Total ROS suppression (AUC) when normalized to PEG values represent the means of triplicates ± SE of >three independent experiments. *Difference compared to untreated cells, p < 0.001, ###Difference when compared with untreated cells, p < 0.0001. (c) Unstimulated neutrophils were pre-incubated with free RGD peptides (50 μg/mL) and seeded on RGD containing hydrogels or PEG alone. *Difference when compared with untreated cells (without free RGD) on RGD, p < 0.001. (d) Total ROS (AUC) induced by free RGD ligation. *Difference compared with PEG, p < 0.05, #Difference when compared with untreated cells (without free RGD), p < 0.05, ##p < 0.01
Figure 5
p38 MAPK activation from ECM peptide adherent neutrophils. (a) Western blots of p38 MAPK activity in isolated neutrophils seeded on Fg-coated coverslips, PEG, P2 and RGD peptide hydrogels following 0, 30, 60, 90, or 120 min incubation at 37 °C in sealed chambers. (b) Quantitative measurement of phospho-p38 MAPK. Western blots were performed in triplicate. Analysis conducted with Image Studio Lite software intensity measurements and compared to β-actin for each individual blot. **Difference when compared with cells on Fg, RGD and P2, p < 0.001, #Difference between RGD adherent cells and cells attached to Fg, p < 0.01, DifferencebetweenP2adherentcellsandcellsattachedtoFg,p<0.01,Difference between P2 adherent cells and cells attached to Fg, p < 0.01, DifferencebetweenP2adherentcellsandcellsattachedtoFg,p<0.01,$p < 0.001
Figure 6
Schematic representation of αVβ3 integrin ligation and adhesion-mediated ROS suppression of neutrophils. The ROS suppression from adhesive neutrophils is regulated by multiple intracellular molecules through extracelluar integrin ligation. This inhibitory effect in ROS production was distinctively observed as an effect of αVβ3 integrin ligation
Similar articles
- Beta1 integrin activation on human neutrophils promotes beta2 integrin-mediated adhesion to fibronectin.
van den Berg JM, Mul FP, Schippers E, Weening JJ, Roos D, Kuijpers TW. van den Berg JM, et al. Eur J Immunol. 2001 Jan;31(1):276-84. doi: 10.1002/1521-4141(200101)31:1<276::AID-IMMU276>3.0.CO;2-D. Eur J Immunol. 2001. PMID: 11265644 - Transendothelial migration enhances integrin-dependent human neutrophil chemokinesis.
Gonzalez AL, El-Bjeirami W, West JL, McIntire LV, Smith CW. Gonzalez AL, et al. J Leukoc Biol. 2007 Mar;81(3):686-95. doi: 10.1189/jlb.0906553. Epub 2006 Dec 12. J Leukoc Biol. 2007. PMID: 17164427 - Soluble CD40 ligand stimulates CD40-dependent activation of the β2 integrin Mac-1 and protein kinase C zeda (PKCζ) in neutrophils: implications for neutrophil-platelet interactions and neutrophil oxidative burst.
Jin R, Yu S, Song Z, Zhu X, Wang C, Yan J, Wu F, Nanda A, Granger DN, Li G. Jin R, et al. PLoS One. 2013 Jun 6;8(6):e64631. doi: 10.1371/journal.pone.0064631. Print 2013. PLoS One. 2013. PMID: 23785403 Free PMC article. - Integrins in cell adhesion and signaling.
Akiyama SK. Akiyama SK. Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review. - Neutrophil Integrins and Matrix Ligands and NET Release.
O'Brien XM, Reichner JS. O'Brien XM, et al. Front Immunol. 2016 Sep 19;7:363. doi: 10.3389/fimmu.2016.00363. eCollection 2016. Front Immunol. 2016. PMID: 27698655 Free PMC article. Review.
Cited by
- IRF3 Inhibits Neutrophil Recruitment in Mice Infected with Pseudomonas aeruginosa.
Piao Z, Yuan H, Wang C, Shi L. Piao Z, et al. Inflammation. 2017 Jun;40(3):735-744. doi: 10.1007/s10753-017-0517-5. Inflammation. 2017. PMID: 28181039 - Targeting Neutrophil β2-Integrins: A Review of Relevant Resources, Tools, and Methods.
Conley HE, Sheats MK. Conley HE, et al. Biomolecules. 2023 May 26;13(6):892. doi: 10.3390/biom13060892. Biomolecules. 2023. PMID: 37371473 Free PMC article. Review. - Clearance of apoptotic cells by neutrophils in inflammation and cancer.
Ramos C, Oehler R. Ramos C, et al. Cell Death Discov. 2024 Jan 13;10(1):26. doi: 10.1038/s41420-024-01809-7. Cell Death Discov. 2024. PMID: 38218739 Free PMC article. Review. - Neutrophil Migration Is Mediated by VLA-6 in the Inflamed Adipose Tissue.
Lim H, Choe YH, Lee J, Kim GE, Hyun JW, Hyun YM. Lim H, et al. Immune Netw. 2024 May 31;24(3):e23. doi: 10.4110/in.2024.24.e23. eCollection 2024 Jun. Immune Netw. 2024. PMID: 38974215 Free PMC article. - Role of the renin-angiotensin system in NETosis in the coronavirus disease 2019 (COVID-19).
Zhang Q, Ling S, Hu K, Liu J, Xu JW. Zhang Q, et al. Biomed Pharmacother. 2022 Apr;148:112718. doi: 10.1016/j.biopha.2022.112718. Epub 2022 Feb 14. Biomed Pharmacother. 2022. PMID: 35176710 Free PMC article. Review.
References
- Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. Role of p38-mitogen-activated protein kinase in spontaneous apoptosis of human neutrophils. J. Immunol. 1999;162:1692–1700. - PubMed
- Asman B, Strand V, Bylin G, Bergstrom K. Peripheral neutrophils after allergic asthmatic reactions. Int. J. Clin. Lab. Res. 1997;27:185–188. - PubMed
- Baldridge CW, Gerard RW. The extra respiration of phagocytosis. Am. J. Physiol. 1933;103:235–236.
- Balliet RM, Capparelli C, Guido C, Pestell TG, Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Chiavarina B, Pestell RG, Howell A, Sotgia F, Lisanti MP. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle. 2011;10:4065–4073. - PMC - PubMed
- Berman DM, Bosenberg MW, Orwant RL, Thurberg BL, Draetta GF, Fletcher CDM, Loda M. Investigative pathology: leading the post-genomic revolution. Lab. Investig. 2012;92:4–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials