Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans - PubMed (original) (raw)
Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans
Clare Edwards et al. BMC Genet. 2015.
Abstract
Background: Little is known about the role of amino acids in cellular signaling pathways, especially as it pertains to pathways that regulate the rate of aging. However, it has been shown that methionine or tryptophan restriction extends lifespan in higher eukaryotes and increased proline or tryptophan levels increase longevity in C. elegans. In addition, leucine strongly activates the TOR signaling pathway, which when inhibited increases lifespan.
Results: Therefore each of the 20 proteogenic amino acids was individually supplemented to C. elegans and the effects on lifespan were determined. All amino acids except phenylalanine and aspartate extended lifespan at least to a small extent at one or more of the 3 concentrations tested with serine and proline showing the largest effects. 11 of the amino acids were less potent at higher doses, while 5 even decreased lifespan. Serine, proline, or histidine-mediated lifespan extension was greatly inhibited in eat-2 worms, a model of dietary restriction, in daf-16/FOXO, sir-2.1, rsks-1 (ribosomal S6 kinase), gcn-2, and aak-2 (AMPK) longevity pathway mutants, and in bec-1 autophagy-defective knockdown worms. 8 of 10 longevity-promoting amino acids tested activated a SKN-1/Nrf2 reporter strain, while serine and histidine were the only amino acids from those to activate a hypoxia-inducible factor (HIF-1) reporter strain. Thermotolerance was increased by proline or tryptophan supplementation, while tryptophan-mediated lifespan extension was independent of DAF-16/FOXO and SKN-1/Nrf2 signaling, but tryptophan and several related pyridine-containing compounds induced the mitochondrial unfolded protein response and an ER stress response. High glucose levels or mutations affecting electron transport chain (ETC) function inhibited amino acid-mediated lifespan extension suggesting that metabolism plays an important role. Providing many other cellular metabolites to C. elegans also increased longevity suggesting that anaplerosis of tricarboxylic acid (TCA) cycle substrates likely plays a role in lifespan extension.
Conclusions: Supplementation of C. elegans with 18 of the 20 individual amino acids extended lifespan, but lifespan often decreased with increasing concentration suggesting hormesis. Lifespan extension appears to be caused by altered mitochondrial TCA cycle metabolism and respiratory substrate utilization resulting in the activation of the DAF-16/FOXO and SKN-1/Nrf2 stress response pathways.
Figures
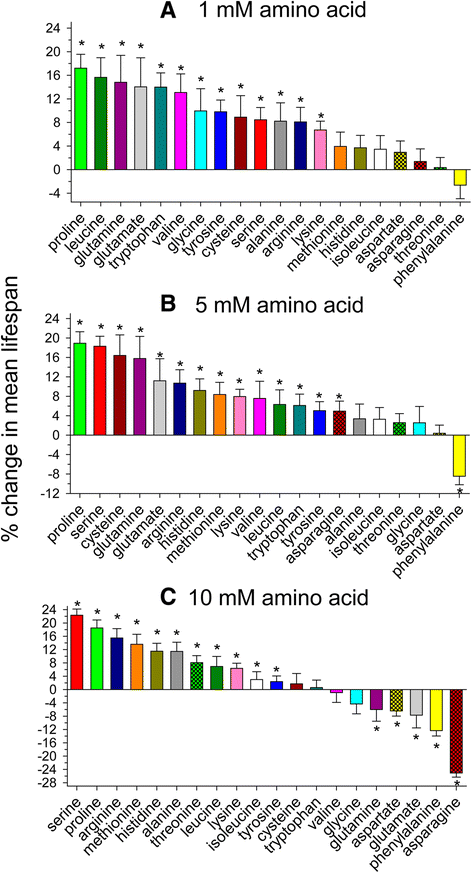
Figure 1
Individual supplementation of most amino acids extends mean lifespan in C. elegans . Mean lifespan of C. elegans supplemented with a (A) 1 mM, (B) 5 mM, or (C) 10 mM concentration of each of the 20 amino acids (* log rank p < 0.05 vs. control).
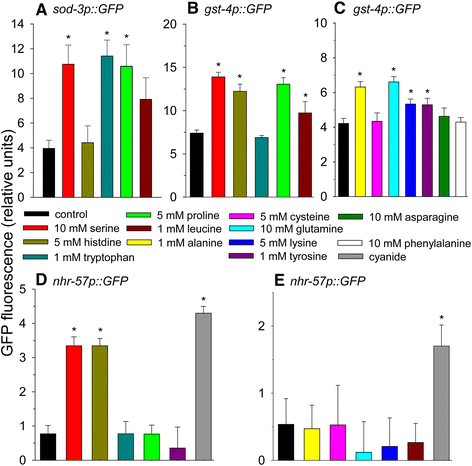
Figure 2
The effects of amino acid addition on DAF-16, SKN-1, and HIF-1-mediated gene expression. (A) The effects of amino acid addition on sod-3p::GFP fluorescence as a measure of DAF-16 transcriptional activity. (B) and (C) The effects of amino acid addition on gst-4p::GFP fluorescence as a measure of SKN-1 transcriptional activity. (D) and (E) The effects of amino acid addition on nhr-57p::GFP fluorescence as a measure of HIF-1 transcriptional activity. 20 μM potassium cyanide was used as a positive control (*p < 0.05).
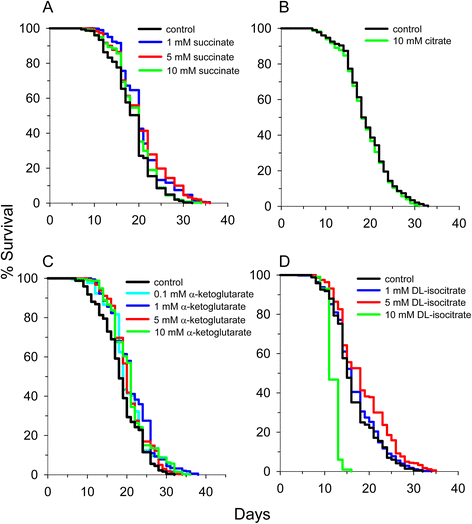
Figure 3
Individual supplementation of many TCA cycle metabolites extends mean lifespan in C. elegans . (A) Succinate extends lifespan, (B) citrate does not extend lifespan, (C) alpha-ketoglutarate extends lifespan, and (D) isocitrate extends lifespan at one or more of the concentrations tested. 10 mM citrate is a standard component of the S-medium. It was removed to determine the effect of citrate on lifespan.
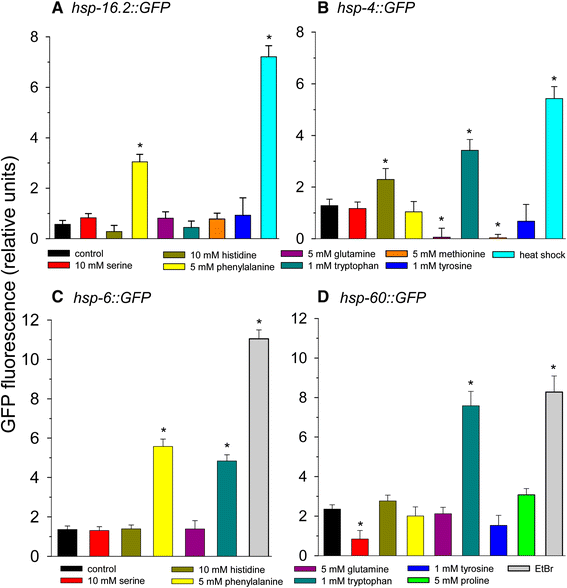
Figure 4
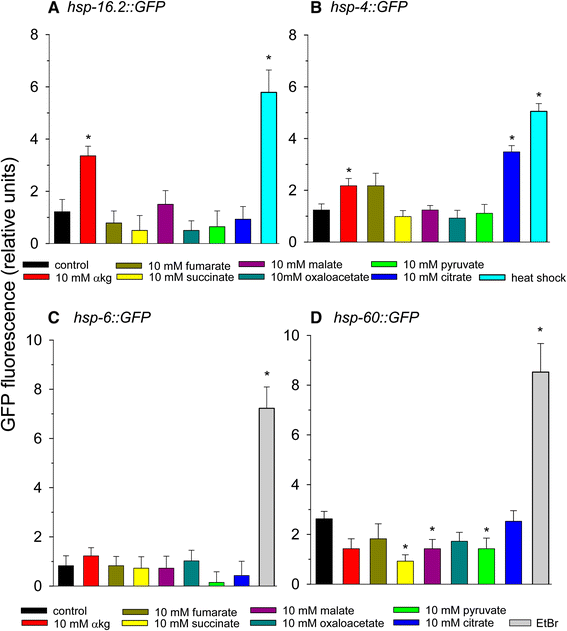
The effects of amino acids on heat shock, mitochondrial unfolded protein response, and ER stress response. Amino acids were added at the concentration tested that yielded maximal lifespan extension except phenylalanine, which did not extend lifespan. (A) Phsp-16.2::GFP (B) Phsp-4::GFP (C) Phsp-6::GFP (D) Phsp-60::GFP. For panels A and B heat shock at 35°C for 2 hours was used as a positive control. For panels C and D 50 μg/ml ethidium bromide treatment for 2 days was used as a positive control (*p < 0.05 vs. control).
Figure 5
The effects of TCA cycle metabolites and pyruvate on heat shock, mitochondrial unfolded protein response, and ER stress response. (A) Phsp-16.2::GFP (B) Phsp-4::GFP (C) Phsp-6::GFP (D) Phsp-60::GFP (*p < 0.05 vs. control). For panels A and B heat shock at 35°C for 2 hours was used as a positive control. For panels C and D 50 μg/ml ethidium bromide treatment for 2 days was used as a positive control (*p < 0.05 vs. control).
Figure 6
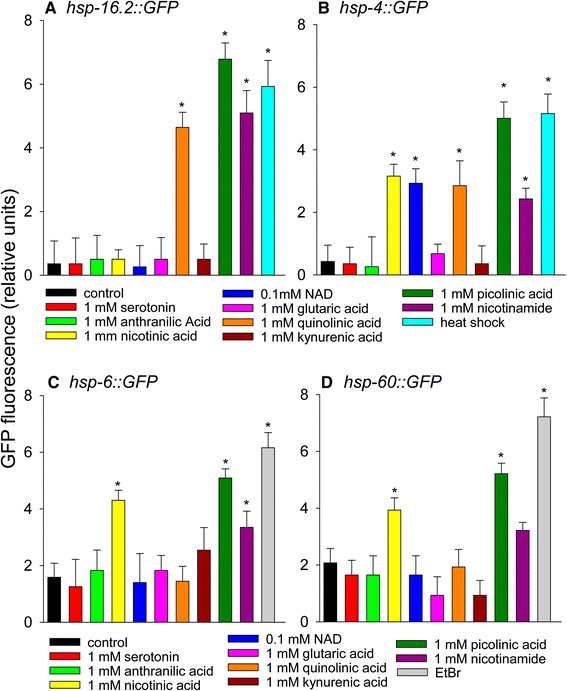
The effects of tryptophan metabolites on heat shock, mitochondrial unfolded protein response, and ER stress response. (A) Phsp-16.2::GFP (B) Phsp-4::GFP (C) Phsp-6::GFP (D) Phsp-60::GFP (*p < 0.05 vs. control). For panels A and B heat shock at 35°C for 2 hours was used as a positive control. For panels C and D 50 μg/ml ethidium bromide treatment for 2 days was used as a positive control (*p < 0.05 vs. control).
Figure 7
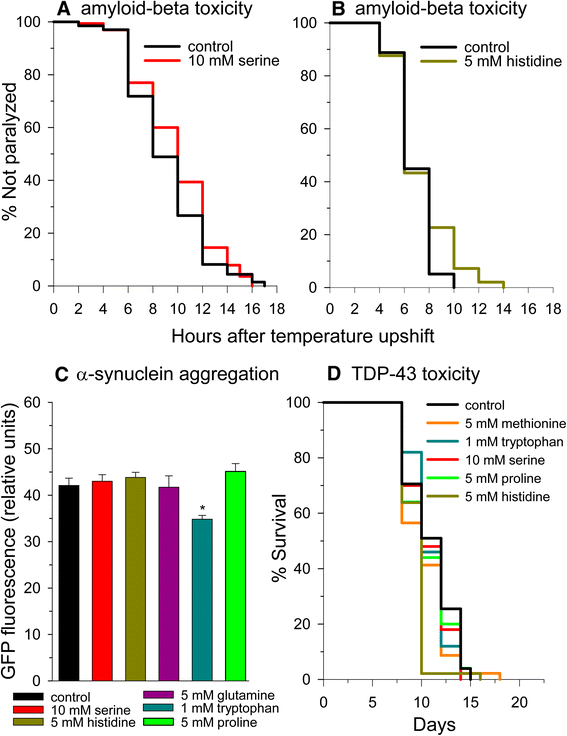
Amino acids do not provide much protection in models of proteotoxicity. (A) Serine and (B) histidine supplementation yield slight protection against muscle amyloid-beta toxicity. (C) Tryptophan supplementation decreases alpha-synuclein aggregate fluorescence. (D) Amino acid supplementation does not protect against TDP-43 toxicity.
Figure 8
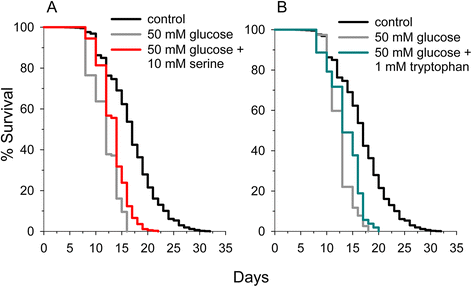
Serine or tryptophan partially prevent high glucose from reducing C. elegans lifespan. (A) Serine extends C. elegans lifespan in the presence of high glucose (p < 0.001). (B) Tryptophan extends C. elegans lifespan in the presence of high glucose (p < 0.001).
Similar articles
- Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-β toxicity in Caenorhabditis elegans: involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1.
Koch K, Weldle N, Baier S, Büchter C, Wätjen W. Koch K, et al. Eur J Nutr. 2020 Feb;59(1):137-150. doi: 10.1007/s00394-019-01894-w. Epub 2019 Feb 1. Eur J Nutr. 2020. PMID: 30710163 - Uric acid induces stress resistance and extends the life span through activating the stress response factor DAF-16/FOXO and SKN-1/NRF2.
Wan QL, Fu X, Dai W, Yang J, Luo Z, Meng X, Liu X, Zhong R, Yang H, Zhou Q. Wan QL, et al. Aging (Albany NY). 2020 Feb 12;12(3):2840-2856. doi: 10.18632/aging.102781. Epub 2020 Feb 12. Aging (Albany NY). 2020. PMID: 32074508 Free PMC article. - Sesamin extends lifespan through pathways related to dietary restriction in Caenorhabditis elegans.
Nakatani Y, Yaguchi Y, Komura T, Nakadai M, Terao K, Kage-Nakadai E, Nishikawa Y. Nakatani Y, et al. Eur J Nutr. 2018 Apr;57(3):1137-1146. doi: 10.1007/s00394-017-1396-0. Epub 2017 Feb 26. Eur J Nutr. 2018. PMID: 28239780 - The search for DAF-16/FOXO transcriptional targets: approaches and discoveries.
Murphy CT. Murphy CT. Exp Gerontol. 2006 Oct;41(10):910-21. doi: 10.1016/j.exger.2006.06.040. Epub 2006 Aug 24. Exp Gerontol. 2006. PMID: 16934425 Review. - 5'-AMP-Activated Protein Kinase Signaling in Caenorhabditis elegans.
Ahmadi M, Roy R. Ahmadi M, et al. Exp Suppl. 2016;107:375-388. doi: 10.1007/978-3-319-43589-3_15. Exp Suppl. 2016. PMID: 27812988 Review.
Cited by
- The role of nutrition and oxidative stress as aging factors in Caenorhabditis elegans.
Yasuda K, Miyazawa M, Ishii T, Ishii N. Yasuda K, et al. J Clin Biochem Nutr. 2023 Nov;73(3):173-177. doi: 10.3164/jcbn.23-44. Epub 2023 Jul 6. J Clin Biochem Nutr. 2023. PMID: 37970544 Free PMC article. - Microbiota-derived metabolites in regulating the development and physiology of Caenorhabditis elegans.
Feng M, Gao B, Garcia LR, Sun Q. Feng M, et al. Front Microbiol. 2023 Feb 28;14:1035582. doi: 10.3389/fmicb.2023.1035582. eCollection 2023. Front Microbiol. 2023. PMID: 36925470 Free PMC article. Review. - 2-hydroxyisobutyric acid (2-HIBA) modulates ageing and fat deposition in Caenorhabditis elegans.
Schifano E, Conta G, Preziosi A, Ferrante C, Batignani G, Mancini P, Tomassini A, Sciubba F, Scopigno T, Uccelletti D, Miccheli A. Schifano E, et al. Front Mol Biosci. 2022 Nov 23;9:986022. doi: 10.3389/fmolb.2022.986022. eCollection 2022. Front Mol Biosci. 2022. PMID: 36533081 Free PMC article. - A Lactobacilli diet that confers MRSA resistance causes amino acid depletion and increased antioxidant levels in the C. elegans host.
Møller KV, Nguyen HTT, Mørch MGM, Hesselager MO, Mulder FAA, Fuursted K, Olsen A. Møller KV, et al. Front Microbiol. 2022 Jul 28;13:886206. doi: 10.3389/fmicb.2022.886206. eCollection 2022. Front Microbiol. 2022. PMID: 35966651 Free PMC article. - The quest to slow ageing through drug discovery.
Partridge L, Fuentealba M, Kennedy BK. Partridge L, et al. Nat Rev Drug Discov. 2020 Aug;19(8):513-532. doi: 10.1038/s41573-020-0067-7. Epub 2020 May 28. Nat Rev Drug Discov. 2020. PMID: 32467649 Review.
References
- Batch BC, Hyland K, Svetkey LP. Branch chain amino acids: biomarkers of health and disease. Curr Opin Clin Nutr Metab Care. 2014;17(1):86–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous