The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility - PubMed (original) (raw)
The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility
William A Comrie et al. J Cell Biol. 2015.
Abstract
Integrity of the dendritic cell (DC) actin cytoskeleton is essential for T cell priming, but the underlying mechanisms are poorly understood. We show that the DC F-actin network regulates the lateral mobility of intracellular cell adhesion molecule 1 (ICAM-1), but not MHCII. ICAM-1 mobility and clustering are regulated by maturation-induced changes in the expression and activation of moesin and α-actinin-1, which associate with actin filaments and the ICAM-1 cytoplasmic domain. Constrained ICAM-1 mobility is important for DC function, as DCs expressing a high-mobility ICAM-1 mutant lacking the cytoplasmic domain exhibit diminished antigen-dependent conjugate formation and T cell priming. These defects are associated with inefficient induction of leukocyte functional antigen 1 (LFA-1) affinity maturation, which is consistent with a model in which constrained ICAM-1 mobility opposes forces on LFA-1 exerted by the T cell cytoskeleton, whereas ICAM-1 clustering enhances valency and further promotes ligand-dependent LFA-1 activation. Our results reveal an important new mechanism through which the DC cytoskeleton regulates receptor activation at the immunological synapse.
© 2015 Comrie et al.
Figures
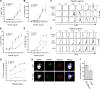
Figure 1.
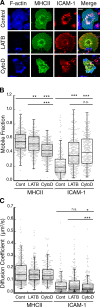
Dendritic cells regulate the lateral mobility of ICAM-1. (A) BMDCs were untreated (broken lines) or treated (solid lines) with 100 ng/ml LPS to induce maturation, stained for the indicated proteins, and analyzed by flow cytometry. Data are representative of three individual experiments. (B) Representative images of BMDCs labeled with Fabs against MHCII or ICAM-1 and imaged at the indicated times after photobleaching at time = 0. Bars, 1 µm. (C and D) Mobile fraction (C) and diffusion coefficient (D) of MHCII and ICAM-1 on the ventral surface of control or LPS-matured BMDCs. Dots represent individual FRAP measurements (n = 102–433) pooled from at least three independent experiments. ***, P < 0.0001.
Figure 2.
The DC actin cytoskeleton clusters ICAM-1 and constrains its mobility. (A) Mature BMDCs were treated with the indicated actin-depolymerizing agents, fixed, and labeled for cell surface MHCII and ICAM-1, followed by permeabilization and labeling for F-actin. Bar, 10 µm. (B and C) Mobile fraction (B) and diffusion coefficient (C) of MHCII and ICAM-1 on mature BMDCs treated as indicated. Dots represent individual FRAP measurements (n = 253–417) pooled from four independent experiments. *, P < 0.01; **, P < 0.001; ***, P < 0.0001.
Figure 3.
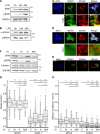
Actin regulatory proteins moesin and α-actinin-1 regulate the lateral mobility of ICAM-1. (A and B) Western blots showing levels of total and phosphorylated ERM proteins (A) and α-actinin1 in lysates from BMDCs matured with 100 ng/ml of LPS for 24 or 48 h (B). E, ezrin; M, moesin. (C and D) Immunofluorescence micrographs showing the distribution of F-actin and moesin with respect to cell surface ICAM-1 (C) or MHC II (D). Bottom panels show enlarged regions indicated by the white boxes. (E) Midplane of cell prepared as in C, demonstrating co-capping of ICAM-1 and moesin. (F) Western blot showing siRNA-mediated knockdown of either moesin (M), α-actinin 1 (A), or both proteins (M/A) in mature BMDCs. (G and H) Mature BMDCs treated with siRNA as in F were surface labeled with Fabs against MHCII or ICAM-1, and FRAP analysis was performed to determine the mobile fraction (G) and diffusion coefficient (H). Dots represent individual FRAP measurements (n = 143–285) pooled from three independent experiments. *, P < 0.01; ***, P < 0.0001. Bars, 10 µm.
Figure 4.
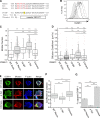
The cytoplasmic tail of ICAM-1 mediates clustering and lateral mobility in the plasma membrane of BMDCs. (A) Sequence alignments of the ICAM-1 cytoplasmic tail and ICAM-1 constructs. (B) ICAM-1−/− BMDCs were transduced with ICAM-1 mutants, and cell surface levels were compared by flow cytometry. ICAM-1−/− cells (shaded gray line), WT cells expressing endogenous ICAM-1 (solid black line), ICAM-1−/− cells reconstituted with WT (solid gray line), Δ Tail (broken gray line), or chimera (broken black line) constructs. Results are representative of three individual experiments. (C and D) Mobility of endogenous ICAM-1 and exogenous ICAM-1 mutants expressed in ICAM-1−/− BMDCs was analyzed using FRAP. (C) Mobile fraction; (D) diffusion coefficient. Dots represent individual FRAP measurements (n = 42–523). Data were pooled from five independent experiments except for endogenous, which was pooled from two experiments, and Y518F, which was from a single experiment. (E) Immunofluorescence microscopy showing the distribution of moesin and F-actin with respect to ICAM-1 in LPS matured ICAM-1−/− BMDCs reconstituted with exogenous WT, Δ Tail, or chimeric ICAM-1. Bars, 10 µm. (F) Images collected as in E were analyzed for ICAM-1 clustering by measuring the coefficient of variation (standard deviation/mean) of surface ICAM-1 intensity. Data are from one experiment (n = 50 cells) representative of three independent experiments. (G) Capping of exogenous ICAM-1 in transduced DCs was quantified from midplane images similar to Fig. 3 E. Data are means ± standard deviation (error bars) from four replicate coverslips (∼50 cells each) in one experiment, representative of two independent experiments. **, P < 0.001; ***, P < 0.0001.
Figure 5.
Altering ICAM-1 mobility perturbs T cell adhesion and priming. (A) ICAM-1−/− DCs were transduced with GFP or the indicated ICAM-1 constructs, pulsed with peptide at the indicated concentrations, and used to prime CD4+ OTII T cells. CD25 surface expression was assessed after 18 h of stimulation. (B) T cells were stimulated as in A and IL-2 secretion was assessed after 18 h using a surface capture assay. (C) CFSE-labeled T cells were stimulated as in A, and CFSE dilution was measured after 96 h to assess proliferation. (D and E) Data were further analyzed to assess the percentage of cells that underwent at least one division (D) and the mean number of divisions of dividing cells (E). Results in A–E are representative of five independent experiments, with data in A, B, D, and E showing mean ± standard deviation (error bars) from triplicate samples in one representative experiment. (F) T cell proliferation was assessed after priming with ICAM-1−/− DCs or ICAM-1−/− DCs transduced with WT ICAM-1 or the signaling-incompetent Y518 mutant. Results are representative of three independent experiments. (G) Conjugate formation was assessed by flow cytometry. Data shown are mean ± standard deviation (error bars) from triplicate samples in one experiment, representative of four individual experiments. (H) Representative midplane images showing T cells interacting with DCs that do or do not display capped ICAM-1. Bars, 10 µm. (I) Conjugates were prepared and imaged as in F. DCs interacting with two T cells were randomly selected and scored for homotypic T cell interactions. Data represent means ± standard deviation (error bars) from four independent experiments, with at least 50 cells each. *, P < 0.05.
Figure 6.
Restriction of ICAM-1 lateral mobility promotes LFA-1 affinity maturation. (A, top) Diagram of LFA-1 conformational states with conformation-specific mAb binding sites. (A, bottom) Schematic showing 293T cell–based artificial APCs used to stimulate T cells, as detailed in the Materials and methods. (B and C) Mobile fraction (B) and diffusion coefficient (C) of ICAM-1 in 293T artificial APCs. Dots represent individual FRAP measurements (n = 140–215) pooled from three independent experiments. (D) Ex vivo human T cells were allowed to interact with artificial APCs lacking ICAM-1 (null) or transduced with WT ICAM-1. Conjugates were fixed and labeled with conformation-specific anti–LFA-1 antibodies. Representative micrographs are shown. Bars, 10 µm. (E and F) Conjugates were prepared as in D. (E) The relative proportion of LFA-1 in the extended conformation was assessed based on the ratio of Kim127:TS2/4 labeling intensity. (F) The relative proportion of LFA-1 in the extended open conformation was assessed based on the ratio of m24:TS2/4 labeling intensity. (G–I) Conjugates were prepared and analyzed as in D–F, except that resting T lymphoblasts were used. H and I show T cells from two different human donors in order to show reproducibility. Dots in E–I represent values from single cells (n = 22–107 cells per condition) pooled from two independent experiments (E and F) or three independent experiments (G–I). *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
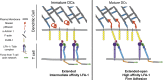
Figure 7.
Model showing how DCs regulate ICAM-1 mobility to enhance LFA-1 activation on T cells. (left) In immature DCs, levels of active moesin and α-actinin are low, allowing significant lateral mobility of ICAM-1. Upon maturation (right), moesin and α-actinin are up-regulated and activated, leading to immobilization of ICAM-1 via interactions between the cytoskeleton and the ICAM-1 cytoplasmic tail. Low-mobility ICAM-1 provides increased resistance to forces applied to the LFA-1 β-chain by the T cell actin cytoskeleton. This process promotes LFA-1 tail separation, and conformational changes in the extracellular domain associated with increased affinity for ICAM-1. Ultimately, these events lead to increased adhesion and T cell activation.
Similar articles
- F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse.
Comrie WA, Babich A, Burkhardt JK. Comrie WA, et al. J Cell Biol. 2015 Feb 16;208(4):475-91. doi: 10.1083/jcb.201406121. Epub 2015 Feb 9. J Cell Biol. 2015. PMID: 25666810 Free PMC article. - Recycling and LFA-1-dependent trafficking of ICAM-1 to the immunological synapse.
Jo JH, Kwon MS, Choi HO, Oh HM, Kim HJ, Jun CD. Jo JH, et al. J Cell Biochem. 2010 Dec 1;111(5):1125-37. doi: 10.1002/jcb.22798. J Cell Biochem. 2010. PMID: 20681010 - WASp-dependent actin cytoskeleton stability at the dendritic cell immunological synapse is required for extensive, functional T cell contacts.
Malinova D, Fritzsche M, Nowosad CR, Armer H, Munro PM, Blundell MP, Charras G, Tolar P, Bouma G, Thrasher AJ. Malinova D, et al. J Leukoc Biol. 2016 May;99(5):699-710. doi: 10.1189/jlb.2A0215-050RR. Epub 2015 Nov 20. J Leukoc Biol. 2016. PMID: 26590149 Free PMC article. - Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors.
Anderson ME, Siahaan TJ. Anderson ME, et al. Peptides. 2003 Mar;24(3):487-501. doi: 10.1016/s0196-9781(03)00083-4. Peptides. 2003. PMID: 12732350 Review. - L-plastin regulates the stability of the immune synapse of naive and effector T-cells.
Wabnitz G, Balta E, Samstag Y. Wabnitz G, et al. Adv Biol Regul. 2017 Jan;63:107-114. doi: 10.1016/j.jbior.2016.09.009. Epub 2016 Sep 27. Adv Biol Regul. 2017. PMID: 27720134 Review.
Cited by
- T Cell engineering for cancer immunotherapy by manipulating mechanosensitive force-bearing receptors.
Zhao L, Zhao G, Feng J, Zhang Z, Zhang J, Guo H, Lin M. Zhao L, et al. Front Bioeng Biotechnol. 2023 Jul 25;11:1220074. doi: 10.3389/fbioe.2023.1220074. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37560540 Free PMC article. Review. - Biophysical Aspects of T Lymphocyte Activation at the Immune Synapse.
Hivroz C, Saitakis M. Hivroz C, et al. Front Immunol. 2016 Feb 15;7:46. doi: 10.3389/fimmu.2016.00046. eCollection 2016. Front Immunol. 2016. PMID: 26913033 Free PMC article. Review. - Soluble CD83 Regulates Dendritic Cell-T Cell Immunological Synapse Formation by Disrupting Rab1a-Mediated F-Actin Rearrangement.
Lin W, Zhou S, Feng M, Yu Y, Su Q, Li X. Lin W, et al. Front Cell Dev Biol. 2021 Jan 22;8:605713. doi: 10.3389/fcell.2020.605713. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33585445 Free PMC article. - Harder, better, faster, stronger: biochemistry and biophysics in the immunosurveillance concert.
Tello-Lafoz M, de Jesus MM, Huse M. Tello-Lafoz M, et al. Trends Immunol. 2022 Feb;43(2):96-105. doi: 10.1016/j.it.2021.12.003. Epub 2021 Dec 29. Trends Immunol. 2022. PMID: 34973924 Free PMC article. Review. - Quantitative proteomic changes in LPS-activated monocyte-derived dendritic cells: A SWATH-MS study.
Arya S, Wiatrek-Moumoulidis D, Synowsky SA, Shirran SL, Botting CH, Powis SJ, Stewart AJ. Arya S, et al. Sci Rep. 2019 Mar 13;9(1):4343. doi: 10.1038/s41598-019-40773-6. Sci Rep. 2019. PMID: 30867486 Free PMC article.
References
- Al-Alwan M.M., Liwski R.S., Haeryfar S.M., Baldridge W.H., Hoskin D.W., Rowden G., and West K.A.. 2003. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J. Immunol. 171:4479–4483 10.4049/jimmunol.171.9.4479 - DOI - PubMed
- Allenspach E.J., Cullinan P., Tong J., Tang Q., Tesciuba A.G., Cannon J.L., Takahashi S.M., Morgan R., Burkhardt J.K., and Sperling A.I.. 2001. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 15:739–750 10.1016/S1074-7613(01)00224-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA093615/CA/NCI NIH HHS/United States
- P01CA093615/CA/NCI NIH HHS/United States
- R01 AI065644/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 GM104867/GM/NIGMS NIH HHS/United States
- GM104867/GM/NIGMS NIH HHS/United States
- R01AI065644/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous