Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses - PubMed (original) (raw)
. 2015 Feb 12;160(4):619-630.
doi: 10.1016/j.cell.2015.01.032.
WenLi Du 2, Marne C Hagemeijer 2, Peter M Takvorian 3, Cyrilla Pau 3, Ann Cali 3, Christine A Brantner 4, Erin S Stempinski 4, Patricia S Connelly 4, Hsin-Chieh Ma 5, Ping Jiang 5, Eckard Wimmer 5, Grégoire Altan-Bonnet 6, Nihal Altan-Bonnet 7
Affiliations
- PMID: 25679758
- PMCID: PMC6704014
- DOI: 10.1016/j.cell.2015.01.032
Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses
Ying-Han Chen et al. Cell. 2015.
Abstract
A central paradigm within virology is that each viral particle largely behaves as an independent infectious unit. Here, we demonstrate that clusters of enteroviral particles are packaged within phosphatidylserine (PS) lipid-enriched vesicles that are non-lytically released from cells and provide greater infection efficiency than free single viral particles. We show that vesicular PS lipids are co-factors to the relevant enterovirus receptors in mediating subsequent infectivity and transmission, in particular to primary human macrophages. We demonstrate that clustered packaging of viral particles within vesicles enables multiple viral RNA genomes to be collectively transferred into single cells. This study reveals a novel mode of viral transmission, where enteroviral genomes are transmitted from cell-to-cell en bloc in membrane-bound PS vesicles instead of as single independent genomes. This has implications for facilitating genetic cooperativity among viral quasispecies as well as enhancing viral replication.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
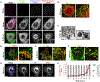
Figure 1.. Poliovirus capsids are captured by phosphatidylserine lipid enriched autophagosome-like organelles and released non-lytically from cells.
(A) Capsids undergo dynamic spatial transitions during infection. HeLa cells infected with PV and immunolabeled with A12, anti-VP1 and anti-3AB antibodies. Scale bars, 5μm. (B) Capsids (A12) colocalized with autophagosome marker LC3-II. PV Infected HeLa cells were immunolabeled with A12 and anti-LC3-II antibodies. Scale bar 5μm. (C) Electron micrographs of PV infected cells show PV capsids in double-membrane autophagosome-like organelles. Scale bars, 5μm and 200nm (Inset). (D) PV infected cells at 4hr p.i. expressing GFP-LactC2 and FAPP1mRFP imaged by structured illumination microscopy. Region of interest is magnified in right panel. Scale bar 5μm. (E) PV infected cells at 7hr p.i. expressing GFP-LactC2 were immunolabeled with anti-GFP and A12 antibodies. Region of interest is magnified in right panel. Arrows indicate A12 positive autophagosome-like organelles co labeled with GFP-LactC2. Scale bar 5μm. (F) Cells co-expressing GFP-LactC2 and LC3-RFP were infected with PV and imaged by confocal microscopy at 7hr pi. Region of interest is magnified in right panel. Scale bar 5μm. (G) Capsid distribution between 7 and 8hr p.i. PV infected cells were immunolabeled with A12, anti-VP1 and anti-3AB antibodies. Scale bar 5μm. (H) Plasma membrane integrity remains intact when PV exits cells. Trypan Blue diffusion across the plasma membrane was measured concurrently with sampling of extracellular medium for PV particles.
Figure 2.. Extracellular PV particles are found in large uniform sized vesicles.
(A) Scanning electron microscopy of a PV infected cell at 7hr p.i.. Scale bar 3um. Inset shows higher magnification of uniform size vesicles docked on the extracellular side of the plasma membrane. Scale bar 1um. (B) Extracellular vesicle size distribution in PV infected cells. Cross section diameter of a 100 randomly selected extracellular vesicles from 4 different cells, were measured from scanning electron micrographs and plotted. Data represented as mean ± SEM. (C) Correlative fluorescence and Scanning Electron Microscopy (SEM). PV infected cell was immunolabeled with A12 at 7hr pi, epifluorescence image was obtained (right) and then sample was processed for SEM (left). Arrows point to A12 labeled extracellular vesicles. Scale bar 1μm.
Figure 3.. Mature PV, CVB3 and Rhinovirus particles are released in phosphatidylserine lipid vesicles.
(A) Cells infected with PV, CVB3 or Rhinovirus were incubated with Alexa568-annexin V and imaged by confocal/DIC at 7hrs pi. Scale bar 5μm for PV and Rhinovirus, 10μm for CVB3. (B) Mock and Apoptotic HeLa cells labeled with Alexa568-Annexin V. Scale bar 10μm. (C) Dynamics of Alexa568-annexin V labeled PS vesicle release from PM projections of PV infected cells at 7hr p.i. (D) Quantification of PS vesicles released from enterovirus infected cells. (E) VP2/VP0 ratio of whole cell lysate and PS vesicles in PV infected cells. Isolated PS vesicles from PV infected cells at 7hr p.i. were analyzed by SDS-PAGE/Western with anti-PV VP2 antibody. (F) Isolated PS vesicles from human Rhinovirus infected cells contain mature Rhinoviral particles. Isolated PS vesicles were processed for SDS-PAGE/Western analysis with anti-HRV2/VP2 (neutralizing) antibodies.
Figure 4.. Infection by PV particles in vesicles is more efficient than free viral particles and is dependent on both the Poliovirus receptor and PS lipids.
(A) Free or vesicle associated PV particles were incubated with new recipient cells and replication was detected at 4hr p.i., by immunolabeling with anti-VP1 antibodies. Scale bar 500μm. (B) CD155/PVR neutralizing antibodies block infection PV particles in vesicles. (C) Blocking PS lipids on vesicles containing PV particles block infection. Vesicles collected by differential centrifugation were incubated with different amounts of Annexin V protein prior to incubation with recipient HeLa cells. Replication was measured at 4hr p.i., by SDS-PAGE/Western analysis with anti-3AB antibody. (D) Quantification of Western results in (C). (E) HeLa cells were incubated with equal titers of free and vesicle associated PV particles. Infection efficiency was determined by quantifying viral 3AB protein levels at peak replication time (4hr p.i.). Data represented as mean ± SD. (F) Primary human macrophages were incubated with equal titers of free and vesicle associated PV particles. Infection efficiency was determined by quantifying viral 3AB protein levels at peak replication time (8hr p.i.). Data represented as mean ± SD.
Figure 5.. PS vesicles contain clustered PV particles, which enable multiple viral RNA genomes to be collectively transferred into a new host cell.
(A) TIRF and DIC images of free and vesicle associated PV particles labeled with Atto488 labeled A12 antibody. (B) Difference in distribution of fluorescence in (A) by computation of the radial autocorrelation function g(r). (C) dSTORM imaging free and vesicle associated viral particles labeled with Atto 488-A12 antibody. (D) Calculation of Ripleys K function to assess the degree of clustering of vesicle associated PV particles relative to free particles. Data represented as mean ± SEM. (E) PS vesicles isolated from PV infected cells using Annexin V microbeads were imaged by TEM. Note the numerous electron dense viral particles in each vesicle and the unilamellar surrounding membrane. Asterisk shows annexin V microbead attached on the exterior of one vesicle. Scale Bars 100 nm. (F) Collected intact vesicles or free viral particles from PV infected cells were incubated with a confluent layer of new host cells at different viral titers. Viral RNAs were monitored by single molecule RNA FISH and imaged by dual confocal/DIC microscopy at 1.5hr p.i. Shown are images of single HeLa cells infected with either free viral particles or vesicle associated viral particles. Images presented were acquired with the same microscopy settings. Scale bar 2μm. (G) Collected vesicles were incubated with cells with/without GuHCL for 1.5hr and viral RNA molecules were monitored by single molecule RNA FISH (see also Figure S3 for quantification). Scale bar 2μm. (H) Quantification of the number of viral RNA molecules per cell in cells infected with either free (n=55 cells) or vesicle associated PV particles (n=55 cells). (***: p<2.10−3; ****: p<10−4) (I) Percent of cells with 15 or greater PV RNA puncta was quantified for cells infected with either free or vesicle-associated PV particles for each viral titer shown. Data represented as mean ± SEM.
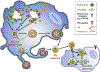
Figure 6.. Model
Assembled mature enteroviruses are released from the replication organelles into the cytoplasm. Clusters of multiple viral particles are selectively captured by double-membraned organelles that originate from the ER and ER derived replication organelles. These double-membraned organelles, which resemble autophagosomes, contain PS lipids on both the lumenal and cytoplasmic leaflets of their membranes. They fuse with the plasma membrane and release a unilamellar PS-lipid enriched vesicle, containing multiple viral particles, into the extracellular medium. This vesicle then facilitates infection in a PS-lipid and viral receptor dependent mechanism resulting in the collective transfer to a new recipient host cell of multiple viral RNA genomes. This mode of viral transmission enhances infection efficiency and potentially allows for genetic complementation among quasispecies.
Similar articles
- Enterovirus Transmission by Secretory Autophagy.
Mutsafi Y, Altan-Bonnet N. Mutsafi Y, et al. Viruses. 2018 Mar 20;10(3):139. doi: 10.3390/v10030139. Viruses. 2018. PMID: 29558400 Free PMC article. Review. - Divergent Requirement for a DNA Repair Enzyme during Enterovirus Infections.
Maciejewski S, Nguyen JH, Gómez-Herreros F, Cortés-Ledesma F, Caldecott KW, Semler BL. Maciejewski S, et al. mBio. 2015 Dec 29;7(1):e01931-15. doi: 10.1128/mBio.01931-15. mBio. 2015. PMID: 26715620 Free PMC article. - Extracellular vesicles: Vehicles of en bloc viral transmission.
Altan-Bonnet N, Perales C, Domingo E. Altan-Bonnet N, et al. Virus Res. 2019 May;265:143-149. doi: 10.1016/j.virusres.2019.03.023. Epub 2019 Mar 27. Virus Res. 2019. PMID: 30928427 Review. - Enterovirus Infection Induces Massive Recruitment of All Isoforms of Small Cellular Arf GTPases to the Replication Organelles.
Moghimi S, Viktorova E, Zimina A, Szul T, Sztul E, Belov GA. Moghimi S, et al. J Virol. 2020 Dec 22;95(2):e01629-20. doi: 10.1128/JVI.01629-20. Print 2020 Dec 22. J Virol. 2020. PMID: 33087467 Free PMC article. - In vitro reconstitution reveals membrane clustering and RNA recruitment by the enteroviral AAA+ ATPase 2C.
Shankar K, Sorin MN, Sharma H, Skoglund O, Dahmane S, Ter Beek J, Tesfalidet S, Nenzén L, Carlson LA. Shankar K, et al. PLoS Pathog. 2024 Aug 5;20(8):e1012388. doi: 10.1371/journal.ppat.1012388. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39102425 Free PMC article.
Cited by
- Extracellular vesicles in virus infection and pathogenesis.
McNamara RP, Dittmer DP. McNamara RP, et al. Curr Opin Virol. 2020 Oct;44:129-138. doi: 10.1016/j.coviro.2020.07.014. Epub 2020 Aug 23. Curr Opin Virol. 2020. PMID: 32846272 Free PMC article. Review. - Mammalian orthoreovirus can exit cells in extracellular vesicles.
Smith SC, Krystofiak E, Ogden KM. Smith SC, et al. PLoS Pathog. 2024 Jan 11;20(1):e1011637. doi: 10.1371/journal.ppat.1011637. eCollection 2024 Jan. PLoS Pathog. 2024. PMID: 38206991 Free PMC article. - A non-enveloped arbovirus released in lysosome-derived extracellular vesicles induces super-infection exclusion.
Labadie T, Roy P. Labadie T, et al. PLoS Pathog. 2020 Oct 19;16(10):e1009015. doi: 10.1371/journal.ppat.1009015. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33075107 Free PMC article. - Extracellular Vesicles: A Novel Mode of Viral Propagation Exploited by Enveloped and Non-Enveloped Viruses.
Chatterjee S, Kordbacheh R, Sin J. Chatterjee S, et al. Microorganisms. 2024 Jan 28;12(2):274. doi: 10.3390/microorganisms12020274. Microorganisms. 2024. PMID: 38399678 Free PMC article. Review. - Illuminating the Sites of Enterovirus Replication in Living Cells by Using a Split-GFP-Tagged Viral Protein.
van der Schaar HM, Melia CE, van Bruggen JA, Strating JR, van Geenen ME, Koster AJ, Bárcena M, van Kuppeveld FJ. van der Schaar HM, et al. mSphere. 2016 Jul 6;1(4):e00104-16. doi: 10.1128/mSphere.00104-16. eCollection 2016 Jul-Aug. mSphere. 2016. PMID: 27390781 Free PMC article.
References
- Borderia AV, Stapleford KA, and Vignuzzi M. (2011) RNA virus population diversityu:implications for interspecies transmission. Curr Opin Virol 1, 643–648 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI083408/AI/NIAID NIH HHS/United States
- AI091985-01A1/AI/NIAID NIH HHS/United States
- R01 AI091985/AI/NIAID NIH HHS/United States
- Z99 HL999999/ImNIH/Intramural NIH HHS/United States
- AI15122/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials