Haematopoietic and immune defects associated with GATA2 mutation - PubMed (original) (raw)
Review
. 2015 Apr;169(2):173-87.
doi: 10.1111/bjh.13317. Epub 2015 Feb 23.
Affiliations
- PMID: 25707267
- PMCID: PMC4409096
- DOI: 10.1111/bjh.13317
Review
Haematopoietic and immune defects associated with GATA2 mutation
Matthew Collin et al. Br J Haematol. 2015 Apr.
Abstract
Heterozygous familial or sporadic GATA2 mutations cause a multifaceted disorder, encompassing susceptibility to infection, pulmonary dysfunction, autoimmunity, lymphoedema and malignancy. Although often healthy in childhood, carriers of defective GATA2 alleles develop progressive loss of mononuclear cells (dendritic cells, monocytes, B and Natural Killer lymphocytes), elevated FLT3 ligand, and a 90% risk of clinical complications, including progression to myelodysplastic syndrome (MDS) by 60 years of age. Premature death may occur from childhood due to infection, pulmonary dysfunction, solid malignancy and MDS/acute myeloid leukaemia. GATA2 mutations include frameshifts, amino acid substitutions, insertions and deletions scattered throughout the gene but concentrated in the region encoding the two zinc finger domains. Mutations appear to cause haplo-insufficiency, which is known to impair haematopoietic stem cell survival in animal models. Management includes genetic counselling, prevention of infection, cancer surveillance, haematopoietic monitoring and, ultimately, stem cell transplantation upon the development of MDS or another life-threatening complication.
Keywords: GATA2; bone marrow failure; immunodeficiency.
© 2015 The Authors. British Journal of Haematology published by John Wiley & Sons Ltd.
Figures
Figure 1
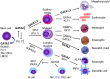
Role of GATA2 in haematopoietic differentiation. Simplified map of key interactions of GATA2 with selected major lineage-specifying transcription factors. The factors indicated are necessary for downstream differentiation according to knock-out models or are highly expressed in differentiated cells but the figure does not contain an exhaustive list of all the transcription factors that have been implicated. Antagonism between pairs of transcription factors is a notable feature of fate decisions, as exemplified by GATA2 and SPI1 (PU.1) in influencing the spectrum of early commitment. In the ‘GATA switch’ GATA2 is replaced by GATA1 at erythroid-specific sites. For more details and source information see: (Orkin, ; Orkin & Zon, ; Bresnick et al, ; Rodrigues et al, 2012).
Figure 2
Gene structure, transcripts and frequency of mutations detected in GATA2. See Table SI for full details. Three GATA2 transcripts have been described in humans: NM_032638.4 (3383 bp) and NM_001145662.1 (3263 bp) both contain six exons using alternative first exons IS and IG, respectively. A third transcript NM_001145661.1 (3484 bp) uses an intervening exon and IG (seven exons) but encodes the same isoform 1 as NM_032638.4 (480 amino acids). NM_001145662.1 is translated into a shorter isoform 2 (466 residues). Transcription of exon IS is haematopoietic specific. Mutations in the fifth intron either affect splicing and cause deletions (1017 + 2 and 1018 − 1) or are localized to the enhancer region (1017 + 512 and 1017 + 572). Annotation of the gene indicates the first base of each exon, numbered from the transcript; annotation of the protein indicates the first and last residue of the zinc fingers (ZF); broken lines show the position of exon boundaries relative to the amino acid sequence. Numbers in parentheses refer to the number of cases with intron mutations.
Figure 3
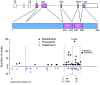
Schematic diagram summarizing the evolution of cellular deficiency in GATA2 mutation. Bone marrow multi-lymphoid progenitors are rapidly lost, even in healthy carriers. Peripheral blood CD34 counts are elevated in many patients and tend to decline with advancing disease. FLT3 ligand is progressively elevated but declines as patients develop MDS. A rapid rise in CD34+ cells and decline in FLT3 ligand may signify the onset of MDS or AML although AML may occur sporadically without prior cytopenia. HPV: human papilloma virus infections; URTI: recurrent bacterial upper respiratory tract infection; FLT3 ligand, fms-related tyrosine kinase 3 ligand; DCML, loss of dendritic cells, monocytes, B and Natural Killer lymphoid cells; MDS, myelodysplastic syndrome; AML, acute myeloid leukaemia.
Similar articles
- Human leukemia mutations corrupt but do not abrogate GATA-2 function.
Katsumura KR, Mehta C, Hewitt KJ, Soukup AA, Fraga de Andrade I, Ranheim EA, Johnson KD, Bresnick EH. Katsumura KR, et al. Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):E10109-E10118. doi: 10.1073/pnas.1813015115. Epub 2018 Oct 9. Proc Natl Acad Sci U S A. 2018. PMID: 30301799 Free PMC article. - Heterogeneity of GATA2-related myeloid neoplasms.
Hirabayashi S, Wlodarski MW, Kozyra E, Niemeyer CM. Hirabayashi S, et al. Int J Hematol. 2017 Aug;106(2):175-182. doi: 10.1007/s12185-017-2285-2. Epub 2017 Jun 22. Int J Hematol. 2017. PMID: 28643018 Review. - GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity.
Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, Cowen EW, Freeman AF, Olivier KN, Uzel G, Zelazny AM, Daub JR, Spalding CD, Claypool RJ, Giri NK, Alter BP, Mace EM, Orange JS, Cuellar-Rodriguez J, Hickstein DD, Holland SM. Spinner MA, et al. Blood. 2014 Feb 6;123(6):809-21. doi: 10.1182/blood-2013-07-515528. Epub 2013 Nov 13. Blood. 2014. PMID: 24227816 Free PMC article. - MDS-associated mutations in germline GATA2 mutated patients with hematologic manifestations.
McReynolds LJ, Yang Y, Yuen Wong H, Tang J, Zhang Y, Mulé MP, Daub J, Palmer C, Foruraghi L, Liu Q, Zhu J, Wang W, West RR, Yohe ME, Hsu AP, Hickstein DD, Townsley DM, Holland SM, Calvo KR, Hourigan CS. McReynolds LJ, et al. Leuk Res. 2019 Jan;76:70-75. doi: 10.1016/j.leukres.2018.11.013. Epub 2018 Dec 4. Leuk Res. 2019. PMID: 30578959 Free PMC article. - GATA2 deficiency.
Hsu AP, McReynolds LJ, Holland SM. Hsu AP, et al. Curr Opin Allergy Clin Immunol. 2015 Feb;15(1):104-9. doi: 10.1097/ACI.0000000000000126. Curr Opin Allergy Clin Immunol. 2015. PMID: 25397911 Free PMC article. Review.
Cited by
- NK Cell Influence on the Outcome of Primary Epstein-Barr Virus Infection.
Chijioke O, Landtwing V, Münz C. Chijioke O, et al. Front Immunol. 2016 Aug 29;7:323. doi: 10.3389/fimmu.2016.00323. eCollection 2016. Front Immunol. 2016. PMID: 27621731 Free PMC article. Review. - Germline Predisposition to Hematolymphoid Neoplasia.
Weinberg OK, Kuo F, Calvo KR. Weinberg OK, et al. Am J Clin Pathol. 2019 Aug 1;152(3):258-276. doi: 10.1093/ajcp/aqz067. Am J Clin Pathol. 2019. PMID: 31309983 Free PMC article. Review. - Impairment of the Hypothalamus-Pituitary-Thyroid Axis Caused by Naturally Occurring GATA2 Mutations In Vitro.
Sakai Y, Ohba K, Sasaki S, Matsushita A, Nakamura HM, Kuroda G, Tsuriya D, Yamashita M, Suda T. Sakai Y, et al. Int J Mol Sci. 2021 Sep 16;22(18):10015. doi: 10.3390/ijms221810015. Int J Mol Sci. 2021. PMID: 34576178 Free PMC article. - GATA2 deficiency and related myeloid neoplasms.
Wlodarski MW, Collin M, Horwitz MS. Wlodarski MW, et al. Semin Hematol. 2017 Apr;54(2):81-86. doi: 10.1053/j.seminhematol.2017.05.002. Epub 2017 May 10. Semin Hematol. 2017. PMID: 28637621 Free PMC article. - Efferocytic Defects in Early Atherosclerosis Are Driven by GATA2 Overexpression in Macrophages.
Yin C, Vrieze AM, Rosoga M, Akingbasote J, Pawlak EN, Jacob RA, Hu J, Sharma N, Dikeakos JD, Barra L, Nagpal AD, Heit B. Yin C, et al. Front Immunol. 2020 Oct 23;11:594136. doi: 10.3389/fimmu.2020.594136. eCollection 2020. Front Immunol. 2020. PMID: 33193444 Free PMC article.
References
- Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, Graf T, Mayfield R, Chan S, Kastner P. Akashi K. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–427. - PubMed
- Ballas ZK, Turner JM, Turner DA, Goetzman EA. Kemp JD. A patient with simultaneous absence of “classical” natural killer cells (CD3−, CD16+, and NKH1+) and expansion of CD3+, CD4−, CD8−, NKH1+ subset. The Journal of Allergy and Clinical Immunology. 1990;85:453–459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous