Identification of age- and disease-related alterations in circulating miRNAs in a mouse model of Alzheimer's disease - PubMed (original) (raw)
Identification of age- and disease-related alterations in circulating miRNAs in a mouse model of Alzheimer's disease
Sylvia Garza-Manero et al. Front Cell Neurosci. 2015.
Abstract
Alzheimer's disease (AD) is a neurodegenerative disorder characterized clinically by the progressive decline of memory and cognition. Histopathologically, two main hallmarks have been identified in AD: amyloid-β peptide extracellular neuritic plaques and neurofibrillary tangles formed by posttranslational modified tau protein. A definitive diagnosis can only be achieved after the post mortem verification of the histological mentioned alterations. Therefore, the development of biomarkers that allow an early diagnosis and/or predict disease progression is imperative. The prospect of a blood-based biomarker is possible with the finding of circulating microRNAs (miRNAs), a class of small non-coding RNAs of 22-25 nucleotides length that regulate mRNA translation rate. miRNAs travel through blood and recent studies performed in potential AD cases suggest the possibility of finding pathology-associated differences in circulating miRNA levels that may serve to assist in early diagnosis of the disease. However, these studies analyzed samples at a single time-point, limiting the use of miRNAs as biomarkers in AD progression. In this study we evaluated miRNA levels in plasma samples at different time-points of the evolution of an AD-like pathology in a transgenic mouse model of the disease (3xTg-AD). We performed multiplex qRT-PCR and compared the plasmatic levels of 84 miRNAs previously associated to central nervous system development and disease. No significant differences were detected between WT and transgenic young mice. However, age-related significant changes in miRNA abundance were observed for both WT and transgenic mice, and some of these were specific for the 3xTg-AD. In agreement, variations in the levels of particular miRNAs were identified between WT and transgenic old mice thus suggesting that the age-dependent evolution of the AD-like pathology, rather than the presence and expression of the transgenes, modifies the circulating miRNA levels in the 3xTg-AD mice.
Keywords: 3x-Tg; Alzheimer models; blood-based biomarker; early diagnosis; neurodegenerative diseases; pathological aging; plasma; prognosis.
Figures
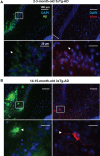
Figure 1
Development of AD-like histopathological features in the 3xTg-AD. Brain coronal sections from the hippocampal CA1 field of 3x-Tg-AD mice. (A) Two- to three-month-old mice sections show Aβ (green) and p-tau (red) human transgene expression. Bottom panels correspond to magnifications of delineated regions in top images; arrows point at Aβ (left) and p-tau (right) immunostaining with no evidence of AD-like histopathological features. (B) Fourteen- to fifteen-month-old mice sections show extracellular aggregates of Aβ (green) and neuronal processes containing p-tau (red). Bottom panels correspond to magnifications of delineated regions in top images; arrowheads point at features indicative of the presence of AD histopathological hallmarks. Scale bars in (A) and (B): 100 μm (top images) and 20 μm (bottom images).
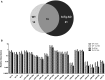
Figure 2
Significant age-related changes in plasmatic miRNAs in WT and 3x-Tg-AD analyzed by qRT-PCR. (A) The Venn diagram depicts the number of miRNAs showing statistically different plasma levels between young and old mice in WT (33 miRNAs, in light gray) and in 3xTg-AD mice (40 miRNAs, in black) as shown by a _t_-test; p < 0.05. From these miRNAs, 19 are common to both comparisons (dark gray). (B) Relative abundance among groups of each of the 19 matching miRNAs. Relative abundance was calculated considering the difference of the Ct of each miRNA and the Ct of the miR-39 of C. elegans mimic (ΔCt) using the formula 2-ΔCt. miR-39 from C. elegans was used as spike-in control in all qRT-PCR experiments. Only miRNAs that showed statistical differences when comparing young and old WT or Tg subjects are shown in the graph; _t_-test; p < 0.05.
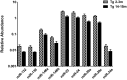
Figure 3
Significant age-dependent changes in plasmatic miRNAs in the 3xTg-AD analyzed by qRT-PCR. The graph depicts the relative abundance (mean ± SD) of each one of the miRNAs that show statistically significant differences (_t_-test; p < 0.05) in plasma levels between the 2–3 and the 14–15 months-old 3xTg-AD mice. Relative abundances were calculated with the difference of the Ct of each miRNA and the Ct of the miR-39 of C. elegans mimics (ΔCt) using the formula 2-ΔCt. Determination of circulating miRNA profiles included plasma samples from six-seven animals per group and a total of four experimental groups were integrated: 2–3 months old-3xTg-AD mice (n = 3), 2–3 months old-WT mice (n = 3), 14–15 months old-3xTg-AD (n = 3), and 14–15 months old-WT mice (n = 3) (n = samples).
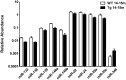
Figure 4
Significant differences in the abundance of plasmatic miRNAs between aging groups analyzed by qRT-PCR. The graph depicts the relative abundance (mean ± SD) of each one of the miRNAs that show statistically significant differences (_t_-test; p < 0.05) in plasma levels between 14–15 months-old WT and 3xTg-AD mice. Relative abundances were calculated with the difference of the Ct of each miRNA and the Ct of the miR-39 of C. elegans mimics (ΔCt) using the formula 2-ΔCt. Determination of circulating miRNA profiles included plasma samples from six-seven animals per group and a total of four experimental groups were integrated: 2–3 months old-3xTg-AD mice (n = 3), 2–3 months old-WT mice (n = 3), 14–15 months old-3xTg-AD (n = 3), and 14–15 months old-WT mice (n = 3) (n = samples).
Similar articles
- Alzheimer's disease: presence and role of microRNAs.
Basavaraju M, de Lencastre A. Basavaraju M, et al. Biomol Concepts. 2016 Aug 1;7(4):241-52. doi: 10.1515/bmc-2016-0014. Biomol Concepts. 2016. PMID: 27505094 Free PMC article. Review. - Molecular and functional signatures in a novel Alzheimer's disease mouse model assessed by quantitative proteomics.
Kim DK, Park J, Han D, Yang J, Kim A, Woo J, Kim Y, Mook-Jung I. Kim DK, et al. Mol Neurodegener. 2018 Jan 16;13(1):2. doi: 10.1186/s13024-017-0234-4. Mol Neurodegener. 2018. PMID: 29338754 Free PMC article. - Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology.
Rosenberg RN, Fu M, Lambracht-Washington D. Rosenberg RN, et al. Alzheimers Res Ther. 2018 Nov 20;10(1):115. doi: 10.1186/s13195-018-0441-4. Alzheimers Res Ther. 2018. PMID: 30454039 Free PMC article. - Peripheral adaptive immunity of the triple transgenic mouse model of Alzheimer's disease.
St-Amour I, Bosoi CR, Paré I, Ignatius Arokia Doss PM, Rangachari M, Hébert SS, Bazin R, Calon F. St-Amour I, et al. J Neuroinflammation. 2019 Jan 5;16(1):3. doi: 10.1186/s12974-018-1380-5. J Neuroinflammation. 2019. PMID: 30611289 Free PMC article. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- Transcriptome analysis of common and diverged circulating miRNAs between arterial and venous during aging.
Wang H, Zhou Y, Yin Z, Chen L, Jin L, Cui Q, Xue L. Wang H, et al. Aging (Albany NY). 2020 Jun 30;12(13):12987-13004. doi: 10.18632/aging.103385. Epub 2020 Jun 30. Aging (Albany NY). 2020. PMID: 32609094 Free PMC article. - Epigenetic mechanisms of neurodegenerative diseases and acute brain injury.
Bertogliat MJ, Morris-Blanco KC, Vemuganti R. Bertogliat MJ, et al. Neurochem Int. 2020 Feb;133:104642. doi: 10.1016/j.neuint.2019.104642. Epub 2019 Dec 12. Neurochem Int. 2020. PMID: 31838024 Free PMC article. Review. - Extracellular microRNAs as messengers in the central and peripheral nervous system.
Scott H. Scott H. Neuronal Signal. 2017 Nov 2;1(4):NS20170112. doi: 10.1042/NS20170112. eCollection 2017 Dec. Neuronal Signal. 2017. PMID: 32714581 Free PMC article. Review. - Alzheimer's disease: presence and role of microRNAs.
Basavaraju M, de Lencastre A. Basavaraju M, et al. Biomol Concepts. 2016 Aug 1;7(4):241-52. doi: 10.1515/bmc-2016-0014. Biomol Concepts. 2016. PMID: 27505094 Free PMC article. Review. - Intrahippocampal Inoculation of Aβ1-42 Peptide in Rat as a Model of Alzheimer's Disease Identified MicroRNA-146a-5p as Blood Marker with Anti-Inflammatory Function in Astrocyte Cells.
Aquino R, de Concini V, Dhenain M, Lam S, Gosset D, Baquedano L, Forero MG, Menuet A, Baril P, Pichon C. Aquino R, et al. Cells. 2023 Feb 22;12(5):694. doi: 10.3390/cells12050694. Cells. 2023. PMID: 36899831 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous