Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models - PubMed (original) (raw)
doi: 10.1038/nm.3843. Epub 2015 May 11.
Beina Teng 2, Changkyu Gu 3, Valentina A Shchedrina 3, Marina Kasaikina 3, Vincent A Pham 3, Nils Hanke 2, Song Rong 2, Faikah Gueler 2, Patricia Schroder 1, Irini Tossidou 2, Joon-Keun Park 2, Lynne Staggs 1, Hermann Haller 1, Sergej Erschow 4, Denise Hilfiker-Kleiner 4, Changli Wei 5, Chuang Chen 5, Nicholas Tardi 5, Samy Hakroush 6, Martin K Selig 7, Aleksandr Vasilyev 8, Sandra Merscher 9, Jochen Reiser 5, Sanja Sever 3
Affiliations
- PMID: 25962121
- PMCID: PMC4458177
- DOI: 10.1038/nm.3843
Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models
Mario Schiffer et al. Nat Med. 2015 Jun.
Abstract
Dysregulation of the actin cytoskeleton in podocytes represents a common pathway in the pathogenesis of proteinuria across a spectrum of chronic kidney diseases (CKD). The GTPase dynamin has been implicated in the maintenance of cellular architecture in podocytes through its direct interaction with actin. Furthermore, the propensity of dynamin to oligomerize into higher-order structures in an actin-dependent manner and to cross-link actin microfilaments into higher-order structures has been correlated with increased actin polymerization and global organization of the actin cytoskeleton in the cell. We found that use of the small molecule Bis-T-23, which promotes actin-dependent dynamin oligomerization and thus increased actin polymerization in injured podocytes, was sufficient to improve renal health in diverse models of both transient kidney disease and CKD. In particular, administration of Bis-T-23 in these renal disease models restored the normal ultrastructure of podocyte foot processes, lowered proteinuria, lowered collagen IV deposits in the mesangial matrix, diminished mesangial matrix expansion and extended lifespan. These results further establish that alterations in the actin cytoskeleton of kidney podocytes is a common hallmark of CKD, while also underscoring the substantial regenerative potential of injured glomeruli and identifying the oligomerization cycle of dynamin as an attractive potential therapeutic target to treat CKD.
Conflict of interest statement
COMPETING FINANCIAL INTEREST
The authors declare competing financial interests: S.S. and J.R. have pending or issued patents on novel kidney-protective therapies that have been out-licensed to Trisaq Inc. in which they have financial interest. In addition, they stand to gain royalties from their commercialization. The remaining authors report no conflicts.
Figures
Figure 1
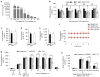
Dynamin oligomerization is essential for kidney function. (a) Phenotype of zebrafish larvae injected with either scrambled (Control MO) or dynamin-2-specific morpholino (dnm2 MO) 120 hours post-fertilization. Scale bars, 2 mm. (b) Survivorship curves of zebrafish larvae injected with either Control MO or dnm2 MO. Each curve represents 180 animals for Control MO and 245 animals for dnm2 MO. Error bars, mean ± SD (log-rank: P < 0.0001 for comparison of mean survival time). (c) Representative image of the fluorescence of circulating eGFP-DBP in the retinal vessel plexus of the fish eye 96 hours post-fertilization and injected with either control MO or dnm2 MO (left) (n = 128 images for control MO, and n = 94 images for dnm2 MO animals). Scale bars, 100 μm. Transmission electron micrographs of glomeruli analyzed in zebrafish larvae 120 hours post-fertilization and injected with either control MO or dnm2 MO (right). Scale bars, 0.5 μm. (d) Intensity of circulating eGFP-DBP (AU, arbitrary units) in the retinal vessel plexus of the fish eye 96 hours post-fertilization and treated with the indicated MO and/or expression construct and with Bis-T-23 (1 ng per larvae) or with DMSO as vehicle (20% per larvae). For groups 1–6, 16, and 20, n = 100–150; for all other groups n = 40–100. Black lines represent median intensity in each group (**P ≤ 0.01, ***P ≤ 0.001, unpaired _t_-test). (e) A schematic diagram indicating the domain structures of dynamin: GTPase, Middle, PH (Pleckstrin-Homology), GED (GTPase Effector Domain), and PRD (Proline/arginine-Rich Domain). Indicated mutations: K/E (K-to-E mutations of the indicated amino acid residues in black), E/K (E-to-K mutations of the indicated residues in red) and I690K. (f) A schematic diagram indicating that dimers of dynamin (DynDIMER) and tetramers of dynamin (DynTETRA) exhibit basal rate of GTP hydrolysis. Oligomerized dynamin (DynOLIGO), whose formation is promoted by Bis-T-23 (structure shown at right) or through indicated mutations, exhibits increased rate of GTP hydrolysis. DynOLIGO induces actin polymerization and crosslinking of F-actin, which in turn regulates the structure and function of podocytes. The small arrows in e and f indicate the effect of the mutations on dynamin’s propensity to oligomerize.
Figure 2
Dynamin oligomerization ameliorates transient proteinuria. (a) Plasma pharmacokinetics of Bis-T-23 after injection (40 mg/kg) in C57BL/6J mice (n = 3) as measured by mass spectrometry. (b) Proteinuria of C57BL/6J mice determined by spot urine test before injection (0 h) and at the indicated hours after injection of the indicated concentrations of Bis-T-23. NS, not statistically significant (unpaired _t-_test; n = 5 mice per concentration). (c-e) Inulin clearance (c), urine volume (d) and para-aminohippurate (PAH) clearance (e) of C57BL/6J mice determined after 8 consecutive days of treatment with DMSO (1%, vehicle) or Bis-T-23 (40 mg/kg). Error bars, mean ± SD (n = 6 mice per condition). (f) The systolic (SYS) or diastolic (DIA) blood pressure of 129X1/SvJ mice measured invasively using a carotid catheter after 8 consecutive days of treatment with DMSO (1%, vehicle) or Bis-T-23 (40 mg/kg) (n = 3 mice per condition). (g) Proteinuria of BALB/c mice determined by spot urine test at indicated times after two consecutive doses of LPS. As indicated, animals were injected with either DMSO (1%, vehicle) or Bis-T-23 (40 mg/kg) (n = 10 mice per condition). Error bars, mean ± SD (*P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001, unpaired _t-_test). (h) Proteinuria of Sprague-Dawley rats treated with PAN and determined by spot urine test. Rats were treated once a day starting 12 days after PAN with DMSO (1%, vehicle) or Bis-T-23 (20 mg/kg) for 6 consecutive days (n = 6 rats per condition). Error bars, mean ± SD (*P ≤ 0.05; ***P ≤ 0.001, unpaired _t-_test).
Figure 3
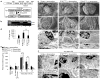
Dynamin oligomerization in podocytes protects against proteinuria. (a) Domain structure of dynamin (top). R725A mutation is situated in the GED, which renders dynamin prone to oligomerize. A schematic diagram (bottom) indicating that the human gene DNM1 carrying R725A mutation (_DNM1_R725A) was placed under the regulation of a tetracycline-responsive promoter element (TRE; tetO). This transgenic mouse (Tg2) was subsequently bred to a second transgenic strain expressing the reverse tetracycline-transactivator (tTA) protein under the control of a podocin-specific promoter to allow for podocyte-specific gene expression (Tg1). Expression of _DNM1_R725A was induced by administration of the tetracycline analog, doxycycline. (b) RT-PCR of DNM1 from wild-type mice (WT), podocin-Cre only transgenic mice (Empty) fed with doxycycline and homozygous _DNM1_R725A/R725A transgenic mice (R725A) fed with doxycycline. Neg, negative control with water as a template; Pos, positive control with plasmid encoding _DNM1_R725A. Nephrin (Nphs1) was used as a positive control. (c) Representative electron micrographs of glomeruli (n = 5–6 glomeruli per genotype) from Empty and R725A transgenic mice fed with either a normal diet (− doxycycline) or doxycycline diet (+ doxycycline). Rows 1 (scale bars, 10 μm), and 2 (scale bars, 1 μm) show scanning electron microscopy. Rows 3 and 4 (scale bars, 1 μm) show transmission electron microscopy (TEM) images. (d) Length of foot processes determined by analyzing images in d. Doxycycline (doxy) (e) Proteinuria determined by analysis of spot urine samples at indicated times and in the indicated genotypes. Mice were fed with doxycycline (doxy) diet before they were injected with LPS (n = 6 mice per condition). Error bars, mean ± SD (***P ≤ 0.001, unpaired _t-_test). (f) Representative TEM images of glomeruli (n = 5–6 glomeruli per genotype) from Empty and R725A transgenic mice 24 hours after LPS injection. Animals were fed with either a normal diet (− doxycycline) or doxycycline diet (+ doxycycline). Scale bars, 2 μm (top row) and 1 μm (bottom row).
Figure 4
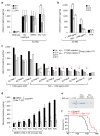
Dynamin oligomerization targets actin cytoskeleton in podocytes. (a) Proteinuria in wild type and ACTN4 mice (without treatment or with treatment with either DMSO (1%, vehicle) or with Bis-T-23 (40 mg/kg)) as determined by spot urine test at indicated time points. Error bars, mean ± SD (**P ≤ 0.01, unpaired _t-_test). (b,c) Proteinuria in ACTN4 mice determined by spot urine test prior to and after double injection of a podocin-driven expression vector encoding DNM1R725A mutant protein. Animals were grouped by protein levels before treatment (n = 3 for > 1,000 μg/ml ACR; n = 7 for 500–1,000 μg/ml ACR). Individual animals from b are shown in c. Red arrows indicate reduction of proteinuria to control levels. Error bars, mean ± SD (**P ≤ 0.01, unpaired _t-_test). (d) Proteinuria in CD2APKO mice determined by spot urine test over several days during which animals were treated daily with DMSO (1%, vehicle) or Bis-T-23 (40 mg/kg), starting at Postnatal Day 18 (n = 5 mice per condition). Error bars, mean ± SD (*P ≤ 0.05; **P ≤ 0.01, ***P ≤ 0.001, unpaired _t-_test). (e) Coomassie blue staining of SDS-PAGE gel showing protein bands from two microliters of mouse spot urine at day 22 in d. BSA was used as a standard. (f) Line graph depicting number of live CD2APKO mice (black lines, n = 20 mice) and CD2APKO mice injected daily with Bis-T-23 (40 mg/kg) (red lines, n = 7 mice) at indicated time points. Animals exhibited a statistically significant difference in survival rate (log-rank: P < 0.0163).
Figure 5
Dymamin oligomerization has beneficial effect on kidney morphology in PKCεKO mice. (a) Proteinuria in PKCεKO mice without treatment or treated with either DMSO (1%, vehicle) or with Bis-T-23 (40 mg/kg) once daily for 8 consecutive days (n = 6 mice per condition) as determined by analysis of the spot urine. Animals were 12 weeks old at the beginning of the treatment (day 0, D0). Error bars, mean ± SD (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; unpaired _t-_test). (b) Total of 150 glomeruli from different animals per group (n = 3) were scored in a blind manner: I = normal glomerulus, II = mild mesangial expansion, III = moderate mesangial expansion, IV = advanced mesangial matrix expansion. (c) Representative images of PAS-stained glomeruli (n = 150 glomeruli per condition) of 14-week-old untreated, DMSO- (1%) and Bis-T-23- (40 mg/kg) treated animals. Scale bars, 20 μm. (d) Representative TEM images of glomeruli (n = 10 glomeruli per group) from wild-type and PKCεKO mice harvested after 8 days of injection with either DMSO (1%, vehicle) or Bis-T-23 (40 mg/kg). Scale bars, 2 μm.
Figure 6
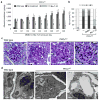
Dynamin oligomerization ameliorates proteinuria due to diabetic nephropathy. (a) Proteinuria in 129X1/SvJ mice determined by analysis of spot urine samples after STZ-induced diabetes. Sixteen weeks after STZ injection (D0), animals were injected with either DMSO (1%, vehicle) or Bis-T-23 (20 mg/kg) once a day for 8 consecutive days (D8) (n = 8 male mice per condition). Error bars, mean ± SD (**P ≤ 0.01; unpaired _t-_test). (b) Representative images of glomeruli stained with PAS (n = 50 glomeruli), stained with Toluidine blue (n = 15 glomeruli), by TEM (n = 15 glomeruli) and by immunofluorescence using anti-collagen IV antibody (n = 50 glomeruli). Glomeruli were isolated from control and diabetic animals treated with either DMSO (1%, vehicle) or Bis-T-23 (40 mg/kg) for 8 days, D8 in a. Scale bars, 20 μm (images showing PAS, Toluidine blue and collagen IV staining) and 5 μm (TEM images). (c) Glomerular collagen IV expression in glomeruli from the indicated mice quantified in a blind manner: 1, very mild; 2 mild; 3, moderate; 4, intense; 5 for very intense. Error bars, mean ± SD (**P ≤ 0.01; unpaired _t-_test). (d) Blood glucose levels in animals used in a. Error bars, mean ± SD. ***P ≤ 0.001; unpaired _t-_test. NS, not statistically significant.
Comment in
- Chronic kidney disease: Actin cytoskeleton alterations in podocytes: a therapeutic target for chronic kidney disease.
Allison SJ. Allison SJ. Nat Rev Nephrol. 2015 Jul;11(7):385. doi: 10.1038/nrneph.2015.79. Epub 2015 May 12. Nat Rev Nephrol. 2015. PMID: 25963588 No abstract available.
Similar articles
- Chronic kidney disease: Actin cytoskeleton alterations in podocytes: a therapeutic target for chronic kidney disease.
Allison SJ. Allison SJ. Nat Rev Nephrol. 2015 Jul;11(7):385. doi: 10.1038/nrneph.2015.79. Epub 2015 May 12. Nat Rev Nephrol. 2015. PMID: 25963588 No abstract available. - Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases.
Sever S, Schiffer M. Sever S, et al. Kidney Int. 2018 Jun;93(6):1298-1307. doi: 10.1016/j.kint.2017.12.028. Epub 2018 Apr 17. Kidney Int. 2018. PMID: 29678354 Free PMC article. Review. - Dynamin Autonomously Regulates Podocyte Focal Adhesion Maturation.
Gu C, Lee HW, Garborcauskas G, Reiser J, Gupta V, Sever S. Gu C, et al. J Am Soc Nephrol. 2017 Feb;28(2):446-451. doi: 10.1681/ASN.2016010008. Epub 2016 Jul 18. J Am Soc Nephrol. 2017. PMID: 27432739 Free PMC article. - Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease.
Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, Henderson J, del Re EC, Hsing L, Erickson A, Cohen CD, Kretzler M, Kerjaschki D, Rudensky A, Nikolic B, Reiser J. Sever S, et al. J Clin Invest. 2007 Aug;117(8):2095-104. doi: 10.1172/JCI32022. J Clin Invest. 2007. PMID: 17671649 Free PMC article. - Drebrin in Renal Glomeruli.
Ludwig-Peitsch WK. Ludwig-Peitsch WK. Adv Exp Med Biol. 2017;1006:337-345. doi: 10.1007/978-4-431-56550-5_20. Adv Exp Med Biol. 2017. PMID: 28865030 Review.
Cited by
- Podocyte-actin dynamics in health and disease.
Perico L, Conti S, Benigni A, Remuzzi G. Perico L, et al. Nat Rev Nephrol. 2016 Nov;12(11):692-710. doi: 10.1038/nrneph.2016.127. Epub 2016 Aug 30. Nat Rev Nephrol. 2016. PMID: 27573725 Review. - Molecular mechanism of Fast Endophilin-Mediated Endocytosis.
Casamento A, Boucrot E. Casamento A, et al. Biochem J. 2020 Jun 26;477(12):2327-2345. doi: 10.1042/BCJ20190342. Biochem J. 2020. PMID: 32589750 Free PMC article. Review. - Lrrk promotes tau neurotoxicity through dysregulation of actin and mitochondrial dynamics.
Bardai FH, Ordonez DG, Bailey RM, Hamm M, Lewis J, Feany MB. Bardai FH, et al. PLoS Biol. 2018 Dec 20;16(12):e2006265. doi: 10.1371/journal.pbio.2006265. eCollection 2018 Dec. PLoS Biol. 2018. PMID: 30571694 Free PMC article. - Novel Markers in Diabetic Kidney Disease-Current State and Perspectives.
Piwkowska A, Zdrojewski Ł, Heleniak Z, Dębska-Ślizień A. Piwkowska A, et al. Diagnostics (Basel). 2022 May 11;12(5):1205. doi: 10.3390/diagnostics12051205. Diagnostics (Basel). 2022. PMID: 35626360 Free PMC article. Review. - Networks that link cytoskeletal regulators and diaphragm proteins underpin filtration function in Drosophila nephrocytes.
Muraleedharan S, Sam A, Skaer H, Inamdar MS. Muraleedharan S, et al. Exp Cell Res. 2018 Mar 15;364(2):234-242. doi: 10.1016/j.yexcr.2018.02.015. Epub 2018 Feb 16. Exp Cell Res. 2018. PMID: 29458174 Free PMC article.
References
- Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. - PubMed
- Saran R, Hedgeman E, Huseini M, Stack A, Shahinian V. Surveillance of chronic kidney disease around the world: tracking and reining in a global problem. Adv Chronic Kidney Dis. 2010;17:271–281. - PubMed
- Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK087985/DK/NIDDK NIH HHS/United States
- R01 DK093773/DK/NIDDK NIH HHS/United States
- R01 DK087985/DK/NIDDK NIH HHS/United States
- P20 GM104318/GM/NIGMS NIH HHS/United States
- R01 DK073495/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous