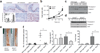Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt - PubMed (original) (raw)
doi: 10.1038/nm.3908. Epub 2015 Jun 24.
Alex S Petrucelli 1, Liang Chen 1, A Alicia Koblansky 1, Agnieszka D Truax 1, Yoshitaka Oyama 1, Arlin B Rogers 2, W June Brickey 1, Yuli Wang 3, Monika Schneider 4, Marcus Mühlbauer 5, Wei-Chun Chou 1, Brianne R Barker 6, Christian Jobin 5, Nancy L Allbritton 3, Dale A Ramsden 7, Beckley K Davis 8, Jenny P Y Ting 1
Affiliations
- PMID: 26107252
- PMCID: PMC4529369
- DOI: 10.1038/nm.3908
Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt
Justin E Wilson et al. Nat Med. 2015 Aug.
Abstract
The inflammasome activates caspase-1 and the release of interleukin-1β (IL-1β) and IL-18, and several inflammasomes protect against intestinal inflammation and colitis-associated colon cancer (CAC) in animal models. The absent in melanoma 2 (AIM2) inflammasome is activated by double-stranded DNA, and AIM2 expression is reduced in several types of cancer, but the mechanism by which AIM2 restricts tumor growth remains unclear. We found that Aim2-deficient mice had greater tumor load than Asc-deficient mice in the azoxymethane/dextran sodium sulfate (AOM/DSS) model of colorectal cancer. Tumor burden was also higher in Aim2(-/-)/Apc(Min/+) than in APC(Min/+) mice. The effects of AIM2 on CAC were independent of inflammasome activation and IL-1β and were primarily mediated by a non-bone marrow source of AIM2. In resting cells, AIM2 physically interacted with and limited activation of DNA-dependent protein kinase (DNA-PK), a PI3K-related family member that promotes Akt phosphorylation, whereas loss of AIM2 promoted DNA-PK-mediated Akt activation. AIM2 reduced Akt activation and tumor burden in colorectal cancer models, while an Akt inhibitor reduced tumor load in Aim2(-/-) mice. These findings suggest that Akt inhibitors could be used to treat AIM2-deficient human cancers.
Figures
Figure 1
AIM2 is distinct from ASC during colitis-associated colon cancer. (a) Induction procedure for the AOM/DSS model of CAC. (b–d) Weight loss (b), % survival (d, days) (c) and average clinical scores (d) in _Aim2_−/− (n = 25), _Asc_−/− (n = 11) and wild-type (n = 30) mice following induction of CAC using AOM/DSS. Mock wild-type animals (n = 10) received a single i.p. injection of AOM and were given regular drinking water instead of DSS. Error bars, s.e.m. (e) H&E staining of colons from AOM/DSS-treated wild-type, _Aim2_−/− and _Asc_−/− mice and (f) semiquantitative scoring of inflammation in colons from AOM/DSS-treated wild-type and _Aim2_−/− mice (n = 6 for WT AOM/Mock and _Aim2_−/− AOM/DSS, and n = 5 for WT AOM/DSS and _Aim2_−/− AOM/Mock). Images were taken at 200× magnification; scale bars, 100 µm. (g) Inflammatory cytokine mRNA (AU, arbitrary units) (n = 3 for WT AOM/Mock and _Aim2_−/− AOM/Mock, and n = 6 for WT AOM/DSS and _Aim2_−/− AOM/DSS) and (h) protein expression in wild-type and _Aim2_−/− colons (n = 5 for WT AOM/Mock and _Aim2_−/− AOM/Mock, n = 10 for WT AOM/DSS and n = 8 for _Aim2_−/− AOM/DSS). (i) Caspase-1 activation in wild-type and _Aim2_−/− colons and (j) IL-1β and IL-18 production in wild-type, _Aim2_−/− and _Asc_−/− colons (n = 5 for WT AOM/Mock, _Aim2_−/− AOM/Mock and _Asc_−/− AOM/Mock, and n = 10 for WT AOM/DSS, n = 8 for _Aim2_−/− AOM/DSS and n = 5 for _Asc_−/− AOM/DSS). Error bars, s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001; b,d,j, one-way ANOVA (Tukey's multiple-comparisons test); c, log-rank test.
Figure 2
AIM2 protects against colon tumorigenesis. (a,b) Mini-endoscopy (a) and representative images (b) of colons from AOM/DSS-treated wild-type (n = 5), _Asc_−/− (n = 3) and _Aim2_−/− mice (n = 5). (c) Macroscopic polyp counts, average polyp size per mouse and tumor load (n = 21 for WT, n = 18 for _Aim2_−/− and n = 10 for _Asc_−/−). (d,e) Semiquantitative scoring of colon hyperplasia (d) and dysplasia (e) in AOM/DSS-treated wild-type and _Aim2_−/− mice (n = 6 for WT AOM/Mock and _Aim2_−/− AOM/DSS, and n = 5 for WT AOM/DSS and _Aim2_−/− AOM/Mock). (f) Colon polyp counts, average polyp size per mouse and tumor load in AOM/DSS-treated wild-type, _Aim2_−/−, _Il1b_−/− and _Aim2_−/−/_Il1b_−/− mice, with representative images of colons in the right panel (n = 5 for wild-type, _Aim2_−/− and _Il1b_−/−, and n = 4 for _Aim2_−/−/_Il1b_−/−). (g) Colon polyp counts, average polyp size per mouse and tumor load in AOM/DSS-treated wild-type and _Aim2_−/− radiation bone marrow chimeras, with representative images of colons in the right panel (n = 4 for WT>WT, n = 5 for WT>_Aim2_−/− and n = 7 for _Aim2_−/−>WT and _Aim2_−/−>_Aim2_−/−). (h) Colon polyp counts, average polyp size per mouse and tumor load in _Apc_Min/+ and _Aim2_−/−/_Apc_Min/+ mice representative images of colons in the right panel (n = 6 mice/group). Each symbol represents one animal. Error bars, s.e.m. ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001; c,g, one-way ANOVA (Tukey's multiple-comparisons test); d,e,f,h, unpaired _t_-test.
Figure 3
AIM2 negatively regulates Akt activity in vitro and in vivo. (a,b) Western blot analyses of PI3K pathway members in the colons of AOM/DSS-treated mice (representative of n = 6 mice/group). (c) Western blot analyses of Akt phosphorylation in colon homogenates from 12-week-old _Apc_Min/+ and _Aim2_−/−/_Apc_Min/+ mice (representative of n = 6 mice/group). (d–g) Western blot analyses of Akt phosphorylation in IGF-1–simulated MEFs from Aim2+/+ and _Aim2_−/− littermates (d), Asc+/+ and _Asc_−/− littermates (e), AIM2–3xFlag- or EV-transfected HCT-116 cells (f), and wild-type and _Aim2_−/− organoids (g). Actin (
β-actin
) and total Akt were used as loading controls. Results are representative of 1 of 3 experiments.
Figure 4
AIM2 restricts cell proliferation and promotes apoptosis. (a) Colon Ki-67 staining in AOM/DSS-treated wild-type and _Aim2_−/− mice (n = 6 for WT AOM/Mock and _Aim2_−/− AOM/DSS, and n = 5 for WT AOM/DSS and _Aim2_−/− AOM/Mock). 200× magnification; scale bars, 100 µm; error bars, s.e.m. (b) Cell numbers of Aim2+/+ and _Aim2_−/− MEFs (d, days). Error bars, s.e.m., from triplicate samples per group. (c) Western blot analyses of activated caspase-7 and procaspase-7 in colons from AOM/DSS-treated wild-type and _Aim2_−/− mice (n = 6 mice/group). (d) Western blot analyses of activated caspase-3 and procaspase-3 in staurosporine (Staur)-treated Aim2+/+ and _Aim2_−/− MEFs (h, hours). Results are representative of 1 of 3 experiments. (e) Representative images of colons from DMSO- or Akt inhibitor (API-2)-treated _Aim2_−/− mice. (f) Number of macroscopic colon polyps, average polyp size and tumor load in API-2-treated _Aim2_−/− mice. Each symbol represents one animal. Error bars denote standard error. ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001. a,b, unpaired _t_-test; f, Mann-Whitney _U_-test (due to the non-normal distribution of the data sets and small sample size).
Figure 5
AIM2 associates with DNA-PK and restricts its activity and Akt phosphorylation. (a) Immunoprecipitation of AIM2 and immunoblots of PI3K members and (b) reciprocal immunoprecipitation of DNA-PKcs and immunoblot of AIM2 in AIM2-Flag (Fg)- or empty vector (EV)-expressing HEK293T cells treated with IGF-1 (IGF) (c) Co-immunoprecipitation of DNA-PKcs and AIM2 in the presence of ethidium bromide (EtBr) in HEK293T cells. (d) Western blot analysis of phosphorylated DNA-PK (p-DNA-PK) in bleocin-treated AIM2-expressing HCT-116 cells. (e) Western blot analysis of p-Akt in 10 µM NU7026- or NU7441-treated Aim2+/+ and _Aim2_−/− MEFs. (f) Western blot analysis of activated caspase-3 in staurosporine (Stauro)-treated Aim2+/+ and _Aim2_−/− MEFs treated with NU7026 (5 µM; h, hours). (g) Cell number of Aim2+/+ and _Aim2_−/− MEFs cultured with 1 µM NU7026 (d, days). Error bars, s.e.m., from triplicate samples per group. Results are representative of 1 of 3 independent experiments (a,b,c,d,e,g) and representative of 1 of 2 independent experiments (f). **P < 0.01, ***P < 0.001. One-way ANOVA (Tukey's multiple-comparisons test).
Similar articles
- The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway.
Ratsimandresy RA, Indramohan M, Dorfleutner A, Stehlik C. Ratsimandresy RA, et al. Cell Mol Immunol. 2017 Jan;14(1):127-142. doi: 10.1038/cmi.2016.35. Epub 2016 Aug 15. Cell Mol Immunol. 2017. PMID: 27524110 Free PMC article. - Macrophage Uptake of Necrotic Cell DNA Activates the AIM2 Inflammasome to Regulate a Proinflammatory Phenotype in CKD.
Komada T, Chung H, Lau A, Platnich JM, Beck PL, Benediktsson H, Duff HJ, Jenne CN, Muruve DA. Komada T, et al. J Am Soc Nephrol. 2018 Apr;29(4):1165-1181. doi: 10.1681/ASN.2017080863. Epub 2018 Feb 9. J Am Soc Nephrol. 2018. PMID: 29439156 Free PMC article. - The DNA Sensor AIM2 Maintains Intestinal Homeostasis via Regulation of Epithelial Antimicrobial Host Defense.
Hu S, Peng L, Kwak YT, Tekippe EM, Pasare C, Malter JS, Hooper LV, Zaki MH. Hu S, et al. Cell Rep. 2015 Dec 1;13(9):1922-36. doi: 10.1016/j.celrep.2015.10.040. Epub 2015 Nov 19. Cell Rep. 2015. PMID: 26655906 Free PMC article. - Immunobiology and structural biology of AIM2 inflammasome.
Wang B, Bhattacharya M, Roy S, Tian Y, Yin Q. Wang B, et al. Mol Aspects Med. 2020 Dec;76:100869. doi: 10.1016/j.mam.2020.100869. Epub 2020 Jul 10. Mol Aspects Med. 2020. PMID: 32660715 Free PMC article. Review. - AIM2 Inflammasome Assembly and Signaling.
Wang B, Tian Y, Yin Q. Wang B, et al. Adv Exp Med Biol. 2019;1172:143-155. doi: 10.1007/978-981-13-9367-9_7. Adv Exp Med Biol. 2019. PMID: 31628655 Review.
Cited by
- AIM2 controls microglial inflammation to prevent experimental autoimmune encephalomyelitis.
Ma C, Li S, Hu Y, Ma Y, Wu Y, Wu C, Liu X, Wang B, Hu G, Zhou J, Yang S. Ma C, et al. J Exp Med. 2021 May 3;218(5):e20201796. doi: 10.1084/jem.20201796. J Exp Med. 2021. PMID: 33710283 Free PMC article. - The crosstalk between immune cells and tumor pyroptosis: advancing cancer immunotherapy strategies.
Hu M, Deng F, Song X, Zhao H, Yan F. Hu M, et al. J Exp Clin Cancer Res. 2024 Jul 10;43(1):190. doi: 10.1186/s13046-024-03115-7. J Exp Clin Cancer Res. 2024. PMID: 38987821 Free PMC article. Review. - Inflammasome signaling in colorectal cancer.
Sharma BR, Kanneganti TD. Sharma BR, et al. Transl Res. 2023 Feb;252:45-52. doi: 10.1016/j.trsl.2022.09.002. Epub 2022 Sep 21. Transl Res. 2023. PMID: 36150688 Free PMC article. Review. - Innate immunity and inflammation.
Xiao TS. Xiao TS. Cell Mol Immunol. 2017 Jan;14(1):1-3. doi: 10.1038/cmi.2016.45. Epub 2016 Aug 22. Cell Mol Immunol. 2017. PMID: 27545072 Free PMC article. No abstract available. - Pyroptosis-Related Signature Predicts the Progression of Ulcerative Colitis and Colitis-Associated Colorectal Cancer as well as the Anti-TNF Therapeutic Response.
Ning Y, Lin K, Fang J, Chen X, Hu X, Liu L, Zhao Q, Wang H, Wang F. Ning Y, et al. J Immunol Res. 2023 Jan 27;2023:7040113. doi: 10.1155/2023/7040113. eCollection 2023. J Immunol Res. 2023. PMID: 36741232 Free PMC article.
References
- Weir HK, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J. Natl. Cancer Inst. 2003;95:1276–1299. - PubMed
- Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. - PubMed
- Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. - PubMed
- Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA156330/CA/NCI NIH HHS/United States
- P01 DK094779/DK/NIDDK NIH HHS/United States
- T32 CA009156/CA/NCI NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- R01 EY024556/EY/NEI NIH HHS/United States
- R01 CA084442/CA/NCI NIH HHS/United States
- R01 AI029564/AI/NIAID NIH HHS/United States
- U19 AI067798/AI/NIAID NIH HHS/United States
- R15 DK098754/DK/NIDDK NIH HHS/United States
- R37 AI029564/AI/NIAID NIH HHS/United States
- NIDDK F32-K088417-01/PHS HHS/United States
- F32 DK088417/DK/NIDDK NIH HHS/United States
- R37-AI029564/AI/NIAID NIH HHS/United States
- F32 DK098916/DK/NIDDK NIH HHS/United States
- U19-AI067798/AI/NIAID NIH HHS/United States
- R01-CA156330/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous