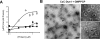A hemi-fission intermediate links two mechanistically distinct stages of membrane fission - PubMed (original) (raw)
. 2015 Aug 6;524(7563):109-113.
doi: 10.1038/nature14509. Epub 2015 Jun 29.
Affiliations
- PMID: 26123023
- PMCID: PMC4529379
- DOI: 10.1038/nature14509
A hemi-fission intermediate links two mechanistically distinct stages of membrane fission
Juha-Pekka Mattila et al. Nature. 2015.
Abstract
Fusion and fission drive all vesicular transport. Although topologically opposite, these reactions pass through the same hemi-fusion/fission intermediate, characterized by a 'stalk' in which only the outer membrane monolayers of the two compartments have merged to form a localized non-bilayer connection. Formation of the hemi-fission intermediate requires energy input from proteins catalysing membrane remodelling; however, the relationship between protein conformational rearrangements and hemi-fusion/fission remains obscure. Here we analysed how the GTPase cycle of human dynamin 1, the prototypical membrane fission catalyst, is directly coupled to membrane remodelling. We used intramolecular chemical crosslinking to stabilize dynamin in its GDP·AlF4(-)-bound transition state. In the absence of GTP this conformer produced stable hemi-fission, but failed to progress to complete fission, even in the presence of GTP. Further analysis revealed that the pleckstrin homology domain (PHD) locked in its membrane-inserted state facilitated hemi-fission. A second mode of dynamin activity, fuelled by GTP hydrolysis, couples dynamin disassembly with cooperative diminishing of the PHD wedging, thus destabilizing the hemi-fission intermediate to complete fission. Molecular simulations corroborate the bimodal character of dynamin action and indicate radial and axial forces as dominant, although not independent, drivers of hemi-fission and fission transformations, respectively. Mirrored in the fusion reaction, the force bimodality might constitute a general paradigm for leakage-free membrane remodelling.
Figures
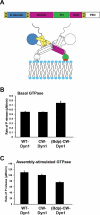
Extended Data Figure 1. Domain structure and biochemical characterization of dynamin constructs
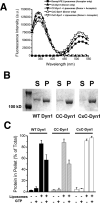
A. Domain structure of dynamin and cartoon illustrating that the GTPase domain (G domain, blue) connects through the bundle signaling element (BSE), composed of the N- and C-terminal helices of the G domain and the C-terminal helix from GED (yellow), to the stalk formed by the middle domain and GED (magenta). The pleckstrin homology domain (PHD, green) interacts with membrane lipids. B. Basal and C. assembly-stimulated rates of GTP hydrolysis rates for 0.5 μM WT and CW-Dyn1 before and after BODIPY conjugation (Data shown as average ±SD, n=3)
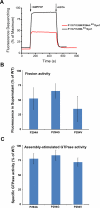
Extended Data Figure 2. Role of P294 in BSE conformational dynamics
A. Changes in emission intensity of BODIPY-labeled CW-Dyn1 and P294A-CW-Dyn1 due to loss of PET following addition of 1 mM GMPPCP. Although the BSE partially opens upon addition of GMPPCP, its movements are constrained relative to WT by the mutation of P294. B. Assembly-stimulated GTPase activity of 0.5 μM P294A, P294G and P294V-Dyn1 measured on 100 nm liposomes relative to WT-Dyn1. The mutants show near WT activity indicating their ability to self-assemble onto and tubulate liposomes (data shown as average ±SD, n=4). C. Fission activity of 0.5 μM P294A, P294G and P294V-Dyn1 relative to WT-Dyn1 measured as the percentage of total membrane released from SUPER templates during 30 min incubation in the presence of GTP (Data shown as average ±SD, n=3). Substitution of P294 with the more rigid valine residue has a greater effect on fission activity.
Extended Data Figure 3. Characterization of CxC-Dyn1
A. Concentration dependence of the specific GTPase hydrolysis rates of WT-Dyn1 (solid squares), CC-Dyn1 (solid circles), and CxC-Dyn1 (open triangles) measured in solution at 1 mM GTP (Data shown as average ±SD, n=3). B. EM micrographs (representative images from 4 independently prepared samples) showing CxC-Dyn1 assembled into rings and short spirals (arrows) in the presence of GMPPCP visualized by negative stain. Insets: top view, rings; side view, short spirals (arrows). Unlike, CxC-Dyn1, CC-Dyn1 remained unassembled in the presence of GMPPCP (data not shown). Scale bars 100 nm.
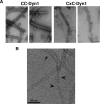
Extended Data Figure 4. Negative stain (A) and cryo-EM (B) images of CC- and CxC-Dyn1 assembled onto PS liposomes in the absence of nucleotides
Note the disordered nature of CxC-Dyn1 spirals relative to CC-Dyn1 structures seen via negative stain in Panel A. Scale bars are 100 nm In panel B arrowhead points to relatively ordered CxC-Dyn1 assemblies while arrowheads point to sparse dynamin assemblies appearing as single or double tings.
Extended Data Figure 5. Altered membrane interactions of the CxC-Dyn1, transition-state conformer
A. Fluorescence emission spectra of 0.1 μM CC-Dyn1 or CxC-Dyn1 (donor) as well as Dansyl-PE (acceptor)-containing liposomes (5 μM total lipid; 90 mol% PS, 10 mol% Dansyl-PE) upon excitation at 280 nm. FRET between the PH domain Trps and Dansyl is evident in the donor+acceptor samples as decrease in donor and increase in acceptor emission. B. Self-assembly of the indicated proteins (1 μM) on liposomes identical to those used in the GTPase assay (300 μM total lipid, Fig. 3F) examined by sedimentation followed by SDS–PAGE analysis of the supernatant (S) and pellet (P) fractions. C. Percentages of proteins pelleted following incubation with or without 400 nm PS liposomes (1 μM protein, 300 μM total lipid) and 1 mM GTP, as indicated, was quantified by sedimentation followed by SDS-PAGE and densitometric analyses of the protein levels in supernatant and pellet fractions (Data shown are average ± Std/ Dev. n=3).
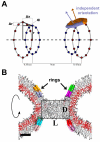
Extended Data Figure 6. Coarse-grained approach to modeling localized membrane constriction by CxC-Dyn1
A. Schematic representation of the geometry of the ring system used to produce local constriction of a prototype membrane tube. Two pairs of rings are shown, each formed by two closely juxtaposed rings (separated by a small distance Δx). The inner ring in each pair has the radius r and the outer ring has a slightly larger radius r+Δr so that the ring pair promotes creation of an hour-glass membrane shape. The PHDs of dynamin are represented as amphiphilic disks evenly distributed over the rings with the center of mass of each disk being restrained to a position on the ring (marked by blue and orange points). Two overlapping disks (purple and brown) attached to the right juxtaposed ring pair are shown. The orientations of the disks are not fixed so the normal to the disk surface (purple and brown arrows) can have an arbitrary direction. B. Axial cross-section of a stable hemi-fission intermediate, the cylindrical micelle, created by the ring system showed in A. The rectangular box indicates the dimensions of the cylindrical micelles (the diameter D and the length L).
Extended Data Figure 7. Molecular simulations of the hemi-fission and fission transformations
The red box shows a representative sequence of simulation snapshots (axial cross-sections) demonstrating the formation of the stable hemi-fission intermediate. Radial constriction of a membrane tube resulted in reversible closure of the tube lumen, i.e. flicker followed by formation of a stable cylindrical micelle structure. The blue box summarizes the simulation runs exploring the stability of the hemi-fission intermediate and its rupture. The upper part shows stable structures corresponding to the constrained intermediate (left, taken at zero tension) and the unconstrained intermediate (right, taken at 0.06 Dyn/cm tension). The lower part shows the rupture of the intermediates by 0.6 Dyn/cm tension (left and right) or by elongation of the ring system at 0.06 Dyn/cm tension (middle). The characteristic times for the rupture are indicated near the corresponding blue arrows.
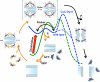
Extended Data Figure 8. Dynamin-catalyzed membrane fission occurs in two mechanistically distinct stages through a hemi-fission intermediate
Model overlaying the distinct dynamin activities and conformational changes onto the two energy barriers (green curve) that must be overcome, first to catalyze formation of the metastable hemi-fission intermediate and subsequently to drive full fission. When trapped in the transition-state and in the absence of GTP, CxC-Dyn1 can drive the formation of a metastable and flickering hemi-fission state (solid blue curve) through the assembly of small scaffolds and enhanced wedging activity of the PHD. However, without subsequent GTPase driven conformational changes required to loosen the scaffold, generate axial force and retract the PHD, as occurs for WT-Dyn1 (dotted blue line), the membrane-bound CxC-Dyn1 creates an insurmountable barrier to fission (dashed blue line).
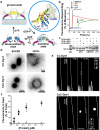
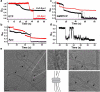
Figure 1. Stabilization of the transition-state conformer of dynamin
a. Cartoon illustrating the mobility of BSE during dynamin's GTPase cycle, blue=apo/GDP-bound, yellow=GMPPCP-bound, yellow/blue=GDP·AlF4--bound transition-state. Inset shows BSE conformation in the crystal structures of GMPPCP- (yellow, PDB: 3ZYC) and GDP·AlF4- (blue, PDB: 2X2E)-bound G domain-BSE fusion protein. b. Loss of PET-dependent quenching of BODIPY fluorescence after addition of GMPPCP, GTP, or GDP either alone, or in the presence of AlCl3 and NaF (i.e. GDP·AlF4-). The decline in fluorescence signal in the presence of GTP reflect its hydrolysis. c. SDS-PAGE of CC-Dyn1 +/− crosslinker. The faster migrating CxC-Dyn1 is stabilized in the transition-state. d. Representative images showing membrane tubulation of SUPER templates (≥5 independent experiments) or GUVs (3 independent experiments) by CC- and CxC-Dyn1 in the absence of nucleotides. Images are inverted for clarity. e. Constriction (seen as dark patches) and fission activity of CC- and CxC-Dyn1 on fluorescently-labeled membrane tethers (see Videos S1 and S2; representative data from 3 independent experiments)) f. Fission activity assessed by vesicle release from SUPER templates of WT- (■) CC- (●), CxC-Dyn1 (△), and CxC-Dyn1 treated with DTT (▽) (average ±SD, n=3).
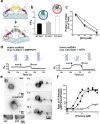
Figure 2. CxC-Dyn1 produces stable hemi-fission
Representative traces of nanotube conductance changes in the presence of CC-Dyn1 (red traces) or CxC-Dyn1 (black traces) obtained in the presence (a) or absence (b) of GTP, or in the presence of GMPPCP (c, d). d. Expanded time-scale of the flickering hemi-fission phenotype, boxed in panel C. Gn indicates conductance normalized to the nanotube conductance before protein addition. e. Cryo-EM images (representative samples from 4 independent experiments) of CxC-Dyn1 assembled on PS liposomes in the presence of GMPPCP. Arrows indicate putative hemi-fission events detected by the loss of a defined inner leaflet of the bilayer occurring at sites of super-constriction (see inset). Scale bars 100 nm.
Figure 3. CxC-Dyn1 displays enhanced membrane wedging activity and altered scaffolding properties
a. Cartoon illustrating transmission of transition-state BSE conformational information through the stalk to the PHD. b. FRET between PHD Trps and Dansyl-lipids measuring the relative membrane insertion of CC- and CxC-Dyn1 (average ±SD, n=3) (see Extended Data Figure 5 for complete spectra). c. Hydrophobic character of membrane insertion of WT-Dyn1 (■), CC-Dyn1 (●), and CxC-Dyn1 (△) measured by resistance to salt extraction. (average ±SD, n=3) d. Differential behavior of nanotubes to vertical displacement of the patch-pipette depending on the nature/persistence of the protein scaffold. Long scaffolds formed by WT or CC-Dyn1 in the presence of GMPPCP prevent retraction of the nanotube into the reservoir when shortened, and hence no change in tube conductance (left panel). Short/flexible scaffolds formed by CxC-Dyn1 in the presence of GTP allow free movement of membranes back into the reservoir, with concomitant increase in conductance (right panel). e. Addition of GTP to GUVs previously tubulated by preassembled CxC-Dyn1 (3 independent experiments) promotes tubule retraction towards the vesicle membrane. The tubules remain constricted during retraction (see Video S3). Images are inverted for clarity. f. Concentration dependence and cooperativity of the assembly-stimulated GTPase activity of WT-Dyn1 (■), CC-Dyn1 (●), and CxC-Dyn1 (△) measured on 100 nm PIP2-containing liposomes (average ±SD, n=3).
Figure 4. The two stages of dynamin-catalyzed membrane fission
a. Coarse-grained simulations revealed formation of a stable hemi-fission intermediate (cylinder-like lipid micelle, middle panel) separating the two different stages of membrane fission. Axial cross-sections of representative snapshots from the simulation runs are shown. Localized radial constriction of a membrane tube by a 2 protein-mimetic ring system (effective radius 5-6 nm, inter-ring distance ~10nm) triggered hemi-fission reaction (red arrow). The tilt characterizes the local membrane orientation imposed by the disks (see Extended Data Fig. 6A for the ring description). The micelle intermediate remained stable under simulation conditions (see Extended Data Fig. 7) unless a moderate axial force was applied to cause its rupture, thus completing the fission reaction (blue arrow). The rectangular box indicates the dimensions of the cylindrical micelle (~9 × 5.5nm). b. Model of distinct dynamin activities and conformational changes mediating the two-stages of dynamin-catalyzed membrane fission that are required to form the metastable hemi-fission intermediate and then to drive full fission (see Extended Data Fig. 8).
Similar articles
- Flexible pivoting of dynamin pleckstrin homology domain catalyzes fission: insights into molecular degrees of freedom.
Baratam K, Jha K, Srivastava A. Baratam K, et al. Mol Biol Cell. 2021 Jul 1;32(14):1306-1319. doi: 10.1091/mbc.E20-12-0794. Epub 2021 May 12. Mol Biol Cell. 2021. PMID: 33979205 Free PMC article. - Cryo-EM of the dynamin polymer assembled on lipid membrane.
Kong L, Sochacki KA, Wang H, Fang S, Canagarajah B, Kehr AD, Rice WJ, Strub MP, Taraska JW, Hinshaw JE. Kong L, et al. Nature. 2018 Aug;560(7717):258-262. doi: 10.1038/s41586-018-0378-6. Epub 2018 Aug 1. Nature. 2018. PMID: 30069048 Free PMC article. - Geometric catalysis of membrane fission driven by flexible dynamin rings.
Shnyrova AV, Bashkirov PV, Akimov SA, Pucadyil TJ, Zimmerberg J, Schmid SL, Frolov VA. Shnyrova AV, et al. Science. 2013 Mar 22;339(6126):1433-6. doi: 10.1126/science.1233920. Science. 2013. PMID: 23520112 Free PMC article. - Dynamin: functional design of a membrane fission catalyst.
Schmid SL, Frolov VA. Schmid SL, et al. Annu Rev Cell Dev Biol. 2011;27:79-105. doi: 10.1146/annurev-cellbio-100109-104016. Epub 2011 May 18. Annu Rev Cell Dev Biol. 2011. PMID: 21599493 Review. - "Gearing" up for dynamin-catalyzed membrane fission.
Khurana H, Pucadyil TJ. Khurana H, et al. Curr Opin Cell Biol. 2023 Aug;83:102204. doi: 10.1016/j.ceb.2023.102204. Epub 2023 Jul 12. Curr Opin Cell Biol. 2023. PMID: 37451176 Review.
Cited by
- Cell biology: Membrane kiss mediates hormone secretion.
Soykan T, Haucke V. Soykan T, et al. Nature. 2016 Jun 23;534(7608):479-80. doi: 10.1038/nature18446. Epub 2016 Jun 15. Nature. 2016. PMID: 27309811 No abstract available. - The role of scaffold reshaping and disassembly in dynamin driven membrane fission.
Pannuzzo M, McDargh ZA, Deserno M. Pannuzzo M, et al. Elife. 2018 Dec 18;7:e39441. doi: 10.7554/eLife.39441. Elife. 2018. PMID: 30561335 Free PMC article. - Dynamic clustering of dynamin-amphiphysin helices regulates membrane constriction and fission coupled with GTP hydrolysis.
Takeda T, Kozai T, Yang H, Ishikuro D, Seyama K, Kumagai Y, Abe T, Yamada H, Uchihashi T, Ando T, Takei K. Takeda T, et al. Elife. 2018 Jan 23;7:e30246. doi: 10.7554/eLife.30246. Elife. 2018. PMID: 29357276 Free PMC article. - Lipid-mediated PX-BAR domain recruitment couples local membrane constriction to endocytic vesicle fission.
Schöneberg J, Lehmann M, Ullrich A, Posor Y, Lo WT, Lichtner G, Schmoranzer J, Haucke V, Noé F. Schöneberg J, et al. Nat Commun. 2017 Jun 19;8:15873. doi: 10.1038/ncomms15873. Nat Commun. 2017. PMID: 28627515 Free PMC article. - SMARTINI3 parametrization of multi-scale membrane models via unsupervised learning methods.
Soleimani A, Risselada HJ. Soleimani A, et al. Sci Rep. 2024 Oct 28;14(1):25714. doi: 10.1038/s41598-024-75490-2. Sci Rep. 2024. PMID: 39468134 Free PMC article.
References
- Schmid SL, Frolov VA. Dynamin: functional design of a membrane fission catalyst. Annu Rev Cell Dev Biol. 2011;27:79–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources