Subcellular localization and RNAs determine FUS architecture in different cellular compartments - PubMed (original) (raw)
. 2015 Sep 15;24(18):5174-83.
doi: 10.1093/hmg/ddv239. Epub 2015 Jun 29.
Affiliations
- PMID: 26123490
- PMCID: PMC4643640
- DOI: 10.1093/hmg/ddv239
Subcellular localization and RNAs determine FUS architecture in different cellular compartments
Liuqing Yang et al. Hum Mol Genet. 2015.
Abstract
Mutations in Fused in sarcoma (FUS) gene cause a subset of familial amyotrophic lateral sclerosis (ALS), a fatal motor neuron degenerative disease. Wild-type FUS is largely localized in the nucleus, but mutant FUS accumulates in the cytoplasm and forms inclusions. It is unclear whether FUS depletion from the nucleus or FUS inclusions in the cytoplasm triggers motor neuron degeneration. In this study, we revealed that the nuclear and cytoplasmic FUS proteins form distinct local distribution patterns. The nuclear FUS forms oligomers and appears granular under confocal microscope. In contrast, the cytoplasmic FUS forms inclusions with no oligomers detected. These patterns are determined by the subcellular localization of FUS, regardless of wild-type or mutant protein. Moreover, mutant FUS remained or re-directed in the nucleus can oligomerize and behave similarly to the wild-type FUS protein. We further found that nuclear RNAs are critical to its oligomerization. Interestingly, the formation of cytoplasmic FUS inclusions is also dependent on RNA binding. Since the ALS mutations disrupt the nuclear localization sequence, mutant FUS is likely retained in the cytoplasm after translation and interacts with cytoplasmic RNAs. We therefore propose that local RNA molecules interacting with the FUS protein in different subcellular compartments play a fundamental role in determining FUS protein architecture and function.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Figure 1.
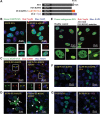
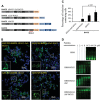
Subcellular localization is the determining factor of FUS distribution patterns. (A) Domain structure of FUS and various FUS constructs used in this study. (B) Full-length wild-type FUS was expressed in HEK cells and formed nuclear granules and was excluded from nucleoli in the nucleus (the left panel). These two features were lost in the truncated FUS ΔQGSY that lacks the QGSY-rich region (the right panel). Nop56 (red) is a marker for nucleoli. The green channel is shown in larger thumbnails and the red and merged images of the same area are shown in smaller thumbnails. (C) The R495X mutant FUS formed cytoplasmic inclusions that are co-localized with the stress granule marker G3BP1 (the left panel). Deleting the QGSY-rich region from R495X did not change the formation of G3BP1-positive cytoplasmic inclusions (the right panel). (D) R521G mutant FUS was expressed in HEK cells and formed both nuclear granules excluded from nucleoli and cytoplasmic inclusions (the left panel). Arrows: nucleoli. Arrow heads: cytoplasmic EGFP-FUS R521G inclusions. Deleting the QGSY-rich region did not change the cytoplasmic inclusions, but changed the distribution of R521G FUS inside the nucleus. The granular pattern disappeared and FUS was evenly distributed in the entire nucleus. (E) Immunostaining of FUS in fibroblast cells derived from familial ALS patients carrying the R521G FUS mutation and healthy controls. Thumbnails are zoomed-in images of two different areas. The solid frame area is zoomed in to show that both wild-type FUS in healthy control cells and R521G mutant FUS in familial ALS patient cells displayed a granular pattern excluded from nucleoli in the nucleus of respective cells (left thumbnails). The dashed frame area is zoomed in with enhanced brightness to show the cytoplasmic retention of R521G mutant FUS in the patient fibroblast cells compared with wild-type FUS in healthy control cells (right thumbnails). Green: FUS; Red: Nop56. (F) FUS R495X formed inclusions in the cytoplasm (the left panel). Tagging FUS R495X with a NLS targeted the chimeric protein in the nucleus (the right panel). The granular pattern of the chimeric protein in the nucleus was similar to wild-type FUS. (G) Tagging the wild-type FUS (the left panel) with a NES caused the cytoplasmic localization of the chimeric protein (the right panel). The wild-type FUS targeted to the cytoplasm also formed inclusions in a similar fashion to mutant FUS in (C) and (D). All images were taken with a Nikon A1 confocal microscope. Green: EGFP-tagged wild-type or mutant FUS. Blue: DAPI staining of DNA.
Figure 2.
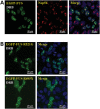
FUS mutants responded to transcription inhibition similarly to wild-type FUS. (A) Wild-type FUS accumulated in areas close to nucleoli in the presence of transcription inhibitor DRB. Cells expressing FUS were treated with 25 μ
m
DRB for 2 h followed by formaldehyde fixation and Nop56 immunostaining. (B) FUS mutants R521G and R495X also accumulated in the peri-nucleolus areas in the presence of DRB. Cells expressing R521G or R495X FUS were treated with 25 μ
m
DRB for 2 h before formaldehyde fixation. All images were taken with a Nikon A1 confocal microscope. Green: EGFP-tagged FUS. Red: Nop56. Blue: DAPI staining of DNA. The distribution of FUS in the absence of DRB was the same as those shown in Figure 1.
Figure 3.
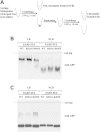
FUS mutants oligomerized similarly to wild-type FUS. (A) Flow chart of preparing CB and NCB samples, see details in Methods. (B) R521G and R495X mutant FUS proteins migrated similarly to wild-type FUS in native gel in both CB and NCB fractions. In the CB fraction, both mutant and wild-type FUS migrated as oligomers. In the NCB fraction, both mutant and wild-type FUS migrated as monomers. (C) The CB and NCB samples were subjected to crosslinking by 1% formaldehyde at room temperature for 10 min. SDS/PAGE analysis showed crosslinked oligomers for both wild-type and mutant FUS in the CB fraction.
Figure 4.
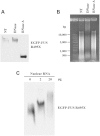
The CB fraction induced oligomerization of FUS in the NCB fraction. (A) Incubation of the CB and NCB fractions induced oligomerization of endogenous wild-type FUS in the NCB fraction. Different amounts of CB samples were incubated with the NCB samples on ice for 20 min. The mixtures were subjected to native gel electrophoresis followed by western blot with a FUS antibody. The individual CB and NCB fractions were shown as control. The slower mobility of FUS suggested a high order assembly of FUS. (B) The R495X mutant FUS also oligomerized when incubated with the CB fraction. The experiment was carried out as in (A) except that R495X mutant was tested in (B).
Figure 5.
Nuclear RNAs triggered FUS oligomerization. (A) Addition of RNase A in the mixture of CB and NCB fractions inhibited the oligomerization of FUS. In contrast, DNase did not have any effect on FUS oligomerization. Fifty units per milliliter RNase-free DNase or 1 ng/ml RNase A was added into mixture of the NCB sample isolated from cells expressing R495X FUS and the CB fraction (1:1 ratio). After 1 h incubation at room temperature, the samples were subjected to native gel electrophoresis and western blot with GFP antibody. (B) Agarose gel electrophoresis followed by ethidium bromide staining was performed to examine the nucleic acid contents in the mixtures from (A). DNA (500–10 000 bp smear) and RNA (visible in the top part of NT lane, >10 000 bp, and in the full DNase lane) were evidently degraded in the presence of DNase and RNase, respectively. (C) The NCB fraction (50 μl) isolated from cells expressing R495X FUS was incubated with 2 or 20 μg of nuclear RNAs extracted with the TRIzol reagent. After incubation at 4°C overnight, the samples were subjected to native gel electrophoresis and western blot.
Figure 6.
RNA binding was also required for the formation of FUS cytoplasmic inclusions. (A) Diagram of GST tagged R495X FUS and a series of truncations lacking RNA-binding motifs. QGSY, the QGSY-rich region; G, the glycine-rich region; RRM, the RNA recognition motif; RGG, arginine–glycine–glycine repeat regions; and ZnF, the zinc finger motif. (B) Inclusion formation of the GST tagged R495X FUS and the RNA-binding motif truncation mutants in HEK cells. GST-FUS mutants were immunostained with a GST antibody and the images were acquired with a Nikon A1 confocal microscope. The insets are magnified areas indicated by yellow squares. Green, GST-FUS; Blue, DAPI staining of DNA. (C) The percentages of inclusion-containing cells with different FUS truncation mutants. More than a hundred cells in three random view fields were counted for each different truncation mutant. Data shown represent mean ± SD and _P_-values were calculated using Student's _t_-test. (D) In vitro RNA binding of FUS truncation mutants with different RNA-binding motifs. Indicated concentrations of purified FUS truncation proteins were incubated with 5′-Cy3-labeled RNA probe and the samples were subjected to polyacrylamide gel electrophoresis (see details in Materials and Methods). The images were acquired with a ProteinSimple gel imaging system. The gel shift of the RNA probe illustrates the binding of FUS protein to the RNA probe.
Figure 7.
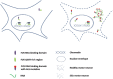
The proposed model of FUS distribution and architecture in different cellular compartments. The left side shows that chromatin-associated RNAs initiate FUS oligomerization through the QGSY-rich region in the nucleus. Nuclear soluble and cytoplasmic FUS normally exists as monomers under physiological conditions. The right side shows that ALS mutations cause cytoplasmic retention of FUS. However, mutant FUS in the nucleus was still able to oligomerize similarly to wild-type FUS. The cytoplasm-retained FUS forms relatively disordered inclusions that require binding cytoplasmic RNAs.
Similar articles
- RNA-binding ability of FUS regulates neurodegeneration, cytoplasmic mislocalization and incorporation into stress granules associated with FUS carrying ALS-linked mutations.
Daigle JG, Lanson NA Jr, Smith RB, Casci I, Maltare A, Monaghan J, Nichols CD, Kryndushkin D, Shewmaker F, Pandey UB. Daigle JG, et al. Hum Mol Genet. 2013 Mar 15;22(6):1193-205. doi: 10.1093/hmg/dds526. Epub 2012 Dec 20. Hum Mol Genet. 2013. PMID: 23257289 Free PMC article. - Self-assembled FUS binds active chromatin and regulates gene transcription.
Yang L, Gal J, Chen J, Zhu H. Yang L, et al. Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):17809-14. doi: 10.1073/pnas.1414004111. Epub 2014 Dec 1. Proc Natl Acad Sci U S A. 2014. PMID: 25453086 Free PMC article. - Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations.
Tradewell ML, Yu Z, Tibshirani M, Boulanger MC, Durham HD, Richard S. Tradewell ML, et al. Hum Mol Genet. 2012 Jan 1;21(1):136-49. doi: 10.1093/hmg/ddr448. Epub 2011 Sep 28. Hum Mol Genet. 2012. PMID: 21965298 - TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: summary and update.
Lattante S, Rouleau GA, Kabashi E. Lattante S, et al. Hum Mutat. 2013 Jun;34(6):812-26. doi: 10.1002/humu.22319. Epub 2013 Apr 29. Hum Mutat. 2013. PMID: 23559573 Review. - How do the RNA-binding proteins TDP-43 and FUS relate to amyotrophic lateral sclerosis and frontotemporal degeneration, and to each other?
Baloh RH. Baloh RH. Curr Opin Neurol. 2012 Dec;25(6):701-7. doi: 10.1097/WCO.0b013e32835a269b. Curr Opin Neurol. 2012. PMID: 23041957 Review.
Cited by
- Yin and yang regulation of stress granules by Caprin-1.
Song D, Kuang L, Yang L, Wang L, Li H, Li X, Zhu Z, Shi C, Zhu H, Gong W. Song D, et al. Proc Natl Acad Sci U S A. 2022 Nov;119(44):e2207975119. doi: 10.1073/pnas.2207975119. Epub 2022 Oct 24. Proc Natl Acad Sci U S A. 2022. PMID: 36279435 Free PMC article. - ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay.
Kamelgarn M, Chen J, Kuang L, Jin H, Kasarskis EJ, Zhu H. Kamelgarn M, et al. Proc Natl Acad Sci U S A. 2018 Dec 18;115(51):E11904-E11913. doi: 10.1073/pnas.1810413115. Epub 2018 Nov 19. Proc Natl Acad Sci U S A. 2018. PMID: 30455313 Free PMC article. - Mechanisms of Long Non-Coding RNAs in the Assembly and Plasticity of Neural Circuitry.
Wang A, Wang J, Liu Y, Zhou Y. Wang A, et al. Front Neural Circuits. 2017 Oct 23;11:76. doi: 10.3389/fncir.2017.00076. eCollection 2017. Front Neural Circuits. 2017. PMID: 29109677 Free PMC article. Review. - Lysine acetylation regulates the RNA binding, subcellular localization and inclusion formation of FUS.
Arenas A, Chen J, Kuang L, Barnett KR, Kasarskis EJ, Gal J, Zhu H. Arenas A, et al. Hum Mol Genet. 2020 Sep 29;29(16):2684-2697. doi: 10.1093/hmg/ddaa159. Hum Mol Genet. 2020. PMID: 32691043 Free PMC article. - FUS interacts with nuclear matrix-associated protein SAFB1 as well as Matrin3 to regulate splicing and ligand-mediated transcription.
Yamaguchi A, Takanashi K. Yamaguchi A, et al. Sci Rep. 2016 Oct 12;6:35195. doi: 10.1038/srep35195. Sci Rep. 2016. PMID: 27731383 Free PMC article.
References
- Kwiatkowski T.J., Jr, Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., et al. (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science, 323, 1205–1208. - PubMed
- Rowland L.P., Shneider N.A. (2001) Amyotrophic lateral sclerosis. N. Engl. J. Med., 344, 1688–1700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous