Mechanism of phospho-ubiquitin-induced PARKIN activation - PubMed (original) (raw)
. 2015 Aug 20;524(7565):370-4.
doi: 10.1038/nature14879. Epub 2015 Jul 10.
Affiliations
- PMID: 26161729
- PMCID: PMC4984986
- DOI: 10.1038/nature14879
Mechanism of phospho-ubiquitin-induced PARKIN activation
Tobias Wauer et al. Nature. 2015.
Erratum in
- Erratum: Mechanism of phospho-ubiquitin-induced PARKIN activation.
Wauer T, Simicek M, Schubert A, Komander D. Wauer T, et al. Nature. 2015 Oct 29;526(7575):728. doi: 10.1038/nature15531. Epub 2015 Sep 23. Nature. 2015. PMID: 26416742 No abstract available.
Abstract
The E3 ubiquitin ligase PARKIN (encoded by PARK2) and the protein kinase PINK1 (encoded by PARK6) are mutated in autosomal-recessive juvenile Parkinsonism (AR-JP) and work together in the disposal of damaged mitochondria by mitophagy. PINK1 is stabilized on the outside of depolarized mitochondria and phosphorylates polyubiquitin as well as the PARKIN ubiquitin-like (Ubl) domain. These phosphorylation events lead to PARKIN recruitment to mitochondria, and activation by an unknown allosteric mechanism. Here we present the crystal structure of Pediculus humanus PARKIN in complex with Ser65-phosphorylated ubiquitin (phosphoUb), revealing the molecular basis for PARKIN recruitment and activation. The phosphoUb binding site on PARKIN comprises a conserved phosphate pocket and harbours residues mutated in patients with AR-JP. PhosphoUb binding leads to straightening of a helix in the RING1 domain, and the resulting conformational changes release the Ubl domain from the PARKIN core; this activates PARKIN. Moreover, phosphoUb-mediated Ubl release enhances Ubl phosphorylation by PINK1, leading to conformational changes within the Ubl domain and stabilization of an open, active conformation of PARKIN. We redefine the role of the Ubl domain not only as an inhibitory but also as an activating element that is restrained in inactive PARKIN and released by phosphoUb. Our work opens up new avenues to identify small-molecule PARKIN activators.
Conflict of interest statement
Statement DK is part of the DUB Alliance that includes Cancer Research Technology and FORMA Therapeutics, and is a consultant for FORMA Therapeutics.
Figures
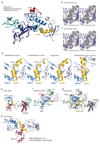
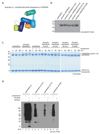
Extended Data Figure 1. Autoinhibited PARKIN and phosphoUb probes
(a) Structure of rat PARKIN (_Rn_PARKIN, pdb-id 4k95, , chain A is used for all representations of _Rn_PARKIN) with Ubl domain coloured in green, the Unique PARKIN domain (UPD) in dark blue, RING1 in blue, IBR in lightblue, REP in red, and RING2 in cyan. Zinc atoms are shown as grey spheres. The catalytic Cys431 is shown in ball-and-stick representation. Disordered linkers are indicated as dotted lines. The three mechanisms of PARKIN inhibition are pointed out in red. (b) Schematic diagram of the closed, autoinhibited conformation of full-length PARKIN. (c) Superposition of the E2~Ub complex from the BIRC7 structure (pdb-id 4auq, 35), superposed via its RING domain onto RING1 of full-length _Rn_PARKIN (pdb-id 4k95, 16) to indicate the position of the E2~Ub on PARKIN RING1. Assuming that E2~Ub adopts a canonical conformation on RING1, the E2 would clash with the Ubl domain and partially with the REP, while the E2-linked Ub would clash with the REP. Hence, Ubl and REP have to be released to enable PARKIN E2~Ub binding at RING1. (d) Time course analysis of an exemplary reaction of UbC3Br (0.2 mg/ml) phosphorylated by GST-_Ph_PINK1 (5 µM) as described previously for ubiquitin. Phosphorylation of proteins was monitored by a band shift on Coomassie-stained Phos-Tag gels. The experiment was performed two times with consistent result. (e) Data collection and refinement statistics.
Extended Data Figure 2. _Ph_PARKIN similarity with human and rat PARKIN and map quality
(a) Structure-based sequence alignment of PARKIN from human (top), rat (middle) and Pediculus humanus corporis (_Ph_PARKIN, bottom). The domains are indicated in boxes coloured according to structural figures. _Hs_PARKIN and _Ph_PARKIN are 45% identical within their crystallised constructs. Secondary structure elements are shown for _Hs_PARKIN Ubl domain (pdb-id 1iyf, 36) and for _Hs_PARKIN core domain (4bm9, 14). Red stars denote the phospho-Ser65 Ub binding pocket, and yellow spheres the residues contacting phosphoUb (see Fig. 2). A black star denotes the catalytic Cys in RING2. (b) Stereo representation of the asymmetric unit of _Ph_PARKIN~pUb crystals, showing 2|Fo|-|Fc| electron density at 1σ, in blue for _Ph_PARKIN and in green for phosphoUb. (c) Electron density detail, shown as in b, zooming in on phosphoUb phospho-Ser65, in stereo representation (d) Superposition of the two _Ph_PARKIN~pUb complexes in the asymmetric unit, coloured as in Fig. 1. The RMSD is 0.76 Å. Electron density is missing for parts of the flexible linker between IBR and RING2. (e) Superposition of available PARKIN structures (4bm9 ; 4i1h ; 4k95 and two _Ph_PARKIN~pUb complexes) in different colours, showing similar domain positions with respect to each other, with exception of the IBR domain. Only the structure of full-length _Rn_PARKIN contains the Ubl domain.
Extended Data Figure 3. Conservation of phosphoUb interacting residues and biochemical analysis
(a-b) An alignment of PARKIN from species available in Ensembl (
) was curated by removing sequences with truncations or poorly sequenced regions. The residues involved in phosphoUb binding, comprising (a) the phosphate pocket and (b) the pUBH are shown, and residues contacting phosphoUb are highlighted. (c) Comparison of residues from _Ph_PARKIN and _Hs_PARKIN involved in phosphoUb binding. Highlighted in green are _Hs_PARKIN β-hairpin residues Gly284 (Gly286 in _Ph_PARKIN) which is mutated to Arg in AR-JP. Other β-hairpin mutations, L283P (Phe285 in _Ph_PARKIN) and H279P (Ser281 in _Ph_PARKIN), introduce Pro residues, likely distorting the β-hairpin loop. (bb), backbone interaction. (d) FP assays performed with mutant PARKIN and phosphoUb. Assays from two independent experiments were combined to produce Fig. 2e. Measurements were performed in triplicate with error bars given as standard deviation from the mean. (e) Coomassie stained SDS-PAGE gel showing _Hs_PARKIN proteins used in activity assays. (f) Normalised proteins for PARKIN activity assays in Fig. 3d. (g) Analogous to Fig. 2a, phosphoUb bound to _Ph_PARKIN is shown with Lys residues in stick representation, and free amine groups as blue spheres. Several Lys side chains (Lys29, Lys33, Lys48 and Lys63) were disordered in the electron density maps, suggesting high flexibility and solvent accessibility, and were modelled in their preferred side chain rotamer for illustrative purposes. When bound to PARKIN, each Lys residue can be ubiquitinated, and the C-terminus which is covalently attached to _Ph_PARKIN in the complex structure, is likely more flexible and could also be attached to a more proximal (phospho)Ub in a chain. This indicates that PARKIN could interact with phosphoUb-containing polyUb chains that were reported to be the PINK1 substrate on mitochondria ,,.
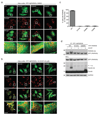
Extended Data Figure 4. PARKIN phosphoUb-binding mutants do not translocate to mitochondria.
(a-b) HeLa cells that do not contain detectable levels of PARKIN were transiently transfected with YFP-PARKIN wt and indicated mutants (green). Tom20 staining with anti-Tom20 antibody indicated mitochondria (red). Images are representative of three biological replicates. (a) In DMSO-treated control cells, PARKIN does not co-localize with mitochondrial Tom20. (b) After treatment with CCCP (10 µM) for 1h, wt PARKIN but not phosphoUb binding mutants co-localize with Tom20. Scale bars, 10 µm. Split channels are shown with overlay to illustrate co-localization. The YFP channel is identical to Fig. 2g. (c) Quantification of cells with PARKIN localized at mitochondria in b, scored for 150 cells per condition in three biological replicates. PhosphoUb binding mutants do not show sustained mitochondrial localization. Error bars represent standard error of the mean. (d) HeLa cells transiently transfected with _Hs_PARKIN wt and mutants were treated with CCCP and YFP-PARKIN immunoprecipitated (see Methods). Whole cell lysates (WCL) or immunoprecipitates (IP) were western blotted for PARKIN, Tom20 and GAPDH (loading control) as indicated. Ubiquitinated forms of PARKIN and Tom20 can be observed with wt PARKIN but not with PARKIN mutants. Also see Fig. 2h where in addition the A320R mutant was included. These experiments were performed three times as biological replicates with similar results. Note that PARKIN autoubiquitination varied and was weaker in some experiments, while Tom20 ubiquitination was more robust. See Supplementary Information for uncropped blots.
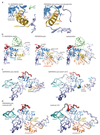
Extended Data Figure 5. The kink in the pUBH.
(a-d) To understand whether the straight pUBH in _Ph_PARKIN was a consequence of a helix-favouring mutation of Gly319 (_Hs_PARKIN) to Ala (Ala321 in _Ph_PARKIN), the _Hs_PARKINΔUbl G319A mutant was crystallised and a structure determined at 2.35 Å resolution (see Extended Data Fig. 1e, Methods). (a) Superposition of _Hs_PARKINΔUbl G319A and wt _Hs_PARKINΔUbl (4bm9, 14). The structures are virtually identical, both containing a kinked helix. (b-c) Electron density detail of the kinked helix for the mutant (b) and wt (c), shown in stereo representation, with 2|Fo|-|Fc| density contoured at 1 σ. The Cβ atom of Ala319 is clearly defined in electron density and does not induce helix straightening in this crystallographic setting. (d) Comparison of pUBH helices in _Hs_PARKINΔUbl G319A (left), _Hs_PARKINΔUbl wt (2nd from left), superposition of the two (2nd from right), and for comparison the straight pUBH in _Ph_PARKIN~pUb. (e-f) Structure of the RBR E3 ligase HHARI (e) in an autoinhibited form (pdb-id 4kbl, 23) showing an entirely different RBR module as compared to full-length _Rn_PARKIN (4k95, 16) in f. RING1, IBR, RING2 domains are coloured as for PARKIN in Fig. 1, and other domains (UBA-like domain and Ariadne domain in HHARI) are coloured in red. Interestingly, the pUBH equivalent helix in HHARI is kinked at a similar position as compared to mammalian PARKIN. (g) Superposition of structures from e and f on their RING1 domains. In HHARI, the pUBH-equivalent helix kinks in a different manner as compared to _Hs_PARKIN. (h) In HHARI, the kinked helix seems to be stabilised by an interaction in cis with the UBA-like domain that binds NEDD8 . The sequence of the helix does not contain Gly residues, but is kinked at a Thr residue (Thr263). It will be interesting to see whether helix straightening occurs in active forms of HHARI that can be induced by binding NEDD8-modified Cullins .
Extended Data Figure 6. Structural detail and B-factor analysis.
(a) PhosphoUb-induced pUBH straightening is energetically neutral as the two hydrogen bonds between helix residues and the RING1 core are not lost but only adjusted. Left, full-length _Rn_PARKIN structure (4k95, 16), right, _Ph_PARKIN~pUb structure. (b) Structure of full-length _Rn_PARKIN (4k95, , left), _Ph_PARKIN~pUb (middle), and a superposition of the two (right), in which Cα atoms of structurally identical, ordered residues of the IBR-REP linker are shown as spheres. The distance between these residues is indicated by a dotted line. The linker sequence is highlighted in Extended Data Fig. 2. (c) Structures of PARKIN in which B-factors were refined for individual atoms (4bm9 ; 4i1h 15) are shown in a ribbon representation, in which the ribbon thickness indicates B-factors differences, with thin ribbons indicating low and thick ribbon indicating high B-factors. The full-length _Rn_PARKIN structure (4k95, 16) is not included since in this 6.5 Å structure overall B-factors were assigned. (d) Structures of _Ph_PARKIN~pUb shown as in c for each molecule of the asymmetric unit. The REP and RING2 elements are destabilised as indicated by higher B-factors. The rigid core of the protein has shifted from the UPD-RING1-RING2 interfaces (compare c) to the UPD-RING1-IBR-phosphoUb interfaces. B-factor analysis may be distorted by neighbouring molecules and crystal contacts, which are not indicated here.
Extended Data Figure 7. The Ile44 patch is essential for PINK1-mediated phosphorylation of Ub and PARKIN Ubl, and controls for Fig. 4c.
All assays were performed three times with consistent results. (a-b) Coomassie stained PhosTag gels comparing the phosphorylation of (a) the _Hs_PARKIN Ubl domain (aa 1-72) and (b) ubiquitin. In both cases, the wt form is compared with the I44A mutant form of the protein. GST-_Tc_PINK1 does not efficiently phosphorylate the I44A mutants of ubiquitin or of the _Hs_PARKIN Ubl domain. This is important since the Ile44 patch in the PARKIN Ubl domain is inaccessible and binds to RING1 in the structure of full-length _Rn_PARKIN (4k95, 16) (see Fig. 4a). (c) Coomassie-stained gel for Fig. 4c with proteins labelled and (d) full-size blot for Fig. 4c.
Extended Data Figure 8. NMR analysis of phospho-Ubl
(a) BEST-TROSY spectra for isotope-labelled _Hs_PARKIN Ubl domain (dark green) and phospho-Ubl domain (light green) with resonances assigned for the Ubl domain. (b) Chemical shift perturbation of Ubl with respect to phospho-Ubl, showing significant perturbations in the region of phosphorylation (Ser65), the last β-strand and neighbouring β-strands. Grey bars, exchange broadened resonances. (c) Mapping of perturbed resonances onto the previously determined NMR structure of _Hs_PARKIN Ubl (1iyf, 36). The perturbed residues cluster in the Ser65-containing loop and in proximity to the Ile44 patch of the Ubl. (d) Mapping of the perturbed resonances to the structure of _Rn_PARKIN (4k95, 16) shows that they perturb the interface between the Ubl domain and the PARKIN core. Thus, phosphorylated Ubl may not be able to (re)bind PARKIN at the same binding site.
Extended Data Figure 9. PARKIN phosphoUbl does not compete with phosphoUb
(a) A possible scenario is that the phosphoUbl competes with phosphoUb for the phosphoUb binding site on PARKIN. This could be favoured since the interaction would occur in cis. (b) Fluorescence polarisation competition experiment increasing the concentration of Ubl, phosphoUbl or phosphoUb with respect to full-length _Hs_PARKIN in the presence of FlAsH-tagged phosphoUb. Measurements were performed in triplicate with error bars given as standard deviation from the mean. While unlabelled phosphoUb competes with labelled phosphoUb in the reaction, Ubl or phosphoUbl do not compete with phosphoUb. (c) Binding of FlAsH-phosphoUb to _Hs_PARKIN and phospho-_Hs_PARKIN. The measurements were performed in the same experiment as samples in Fig. 2d. If phosphoUbl interacts with _Hs_PARKIN in cis, phosphoUb binding should be inhibited. In contrast, binding of phosphoUb to phospho-PARKIN is slightly enhanced as also reported in . Measurements were performed in triplicate and error bars represent standard deviation from the mean. (d) PARKIN phosphorylation assays as in Fig. 4c, including phosphoUbl as well as phosphoUb (both at 10 μM). While addition of phosphoUb induces PARKIN phosphorylation, addition of phosphoUbl does not, indicating that the Ubl is not released from the PARKIN core. The experiment has been performed three times with consistent result.
Extended Data Figure 10. PhosphoUbl-induced ‘opening’ of PARKIN relies on a phosphate pocket in the UPD
(a) A second scenario would be that the phosphorylated Ubl rebinds to PARKIN at an alternative site. We previously speculated that the UPD contains a phosphate-binding site that is lined by two AR-JP patient mutations, K161N and K211N and also contains Arg163 . (b) Full-length _Hs_PARKIN mutants as indicated were phosphorylated in vitro with GST-_Ph_PINK1, resolved on SDS-PAGE and Western blotted using an anti-pSer65 PARKIN antibody (Abcam cat no. ab154995). These proteins were used in c. (c) Ub-vinyl sulphone (Ub-VS) modification of the active site Cys431 of _Hs_PARKIN and _Hs_PARKIN mutants with and without phosphoUb from a time course experiment is assessed on Coomassie stained gels. Ub-VS reacts with ‘open’ forms of PARKIN, and was previously shown to modify phospho-PARKIN but not phosphoUb-activated PARKIN . Phospho-PARKIN mediated ‘opening’ depends on the phosphate pocket present in the UPD, since phospho-PARKIN K211N, K161N (two AR-JP patient mutations) and R163E abrogated or impaired modification by Ub-VS and do not appear to have an accessible catalytic Cys, while the phosphorylated phosphoUb-binding deficient mutant K151E is readily modified. The experiment was performed two times with consistent results and gels have been collated from two different assays (indicated by gap). (d) PARKIN ubiquitination reactions in presence of E1, UBE2L3, Ub or Ub S65A, ATP and GST-_Tc_PINK1 for 2h. PARKIN is activated by phosphoUb or by PARKIN Ubl phosphorylation in absence of phosphoUb (with Ub S65A) (lanes 2/3). Mutants in the UPD phosphate pocket can still be activated by phosphoUb (albeit not to the same extent) (lanes 5, 8, 11) but are inactive when the Ubl is phosphorylated (lanes 6, 9, 12). This could suggest that the phosphoUbl binds back to the PARKIN UPD pocket. However we cannot exclude that e.g. the linker between the Ubl and the UPD plays a more active role in PARKIN activation. The experiment was performed three times with consistent results.
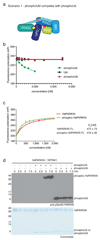
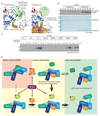
Figure 1. Generation and structure of _Ph_PARKIN-phosphoUb complex.
(a) Schematic of generating phosphoUb suicide probes used in this study. (b) _Hs_PARKINΔUbl (left, aa 137-465) and _Ph_PARKIN (right, aa 140-461) were incubated with indicated Ub suicide probes for 1 h in presence of _Ph_PINK1 (Methods, Extended Data Fig. 1d) and resolved on Coomassie stained SDS-PAGE gels. UbC2Cl, Ub chloroethylamine; UbC2Br, Ub bromoethylamine; UbC3Br, Ub bromopropylamine; UbPrg, Ub propargyl; UbVS, Ub vinylmethylsulphone; UbVME, Ub vinylmethylester. The experiment was performed three times with consistent results. (c) Structure of the _Ph_PARKIN~pUb complex with domains coloured from blue to cyan (UPD, RING1, IBR, RING2), grey zinc atoms, red REP, yellow phosphoUb binding helix (pUBH), and orange phosphoUb. The _C_atalytic Cys in RING2, and key phosphoUb residues are indicated. (d) RMSD values for _Ph_PARKIN in comparison to _Hs_PARKIN (pdb-id 4bm9, 14).
Figure 2. PhosphoUb binding to PARKIN.
(a) PhosphoUb binding site on _Ph_PARKIN as in Fig. 1c. Dotted lines indicate hydrogen bonds. The RING1 β-hairpin that harbours patient mutations, is highlighted in red. bb, backbone contacts. (b) PhosphoUb suicide probe reactions as in Fig. 1b with UbC2Br and GST-_Ph_PINK1. The experiment was performed three times with consistent results. (c) (Left) Occupied Ser65 phosphate pocket in _Ph_PARKIN, (right) identical pocket in _Hs_PARKIN occupied by a sulphate ion in PDB 4bm9 . (d) Fluorescence polarisation experiments characterizing the binding of FlAsH-tagged phosphoUb to PARKIN variants. Measurements were performed in triplicate and error bars represent standard deviation from the mean. mP, milli-polarization unit. (e) Binding assays as in d with full-length _Hs_PARKIN and mutants in the phosphoUb binding site. Binding curves were compiled from experiments shown in Extended Data Fig. 3d. Measurements were performed in triplicate and error bars represent standard deviation from the mean. (f) Activity assays of full-length _Hs_PARKIN variants with and without phosphoUb. After 2 h, reactions were resolved by SDS-PAGE and polyubiquitin visualised by anti-polyubiquitin Western blotting (FK2, Millipore). PARKIN protein normalization is shown in Extended Data Fig. 3e. The experiment was performed three times with consistent results. (g) YFP-_Hs_PARKIN wt or mutants were transfected into HeLa cells, treated with CCCP (10 µM) for 1 h and visualised by immunofluorescence. See Extended Data Fig. 4 for controls and quantification. Scale bar, 10 μm. (h) HeLa cell lysates expressing YFP-_Hs_PARKIN wt or mutants were western blotted for PARKIN (after immunoprecipitation (IP)) and Tom20 (in whole-cell lysate (WCL)). NS, non-specific band. The experiment was performed at least twice as biological replicate for every mutant with consistent results. See Extended Data Fig. 4d and Supplementary Information.
Figure 3. Conformational changes due to phosphoUb binding.
(a) Straight pUBH in _Ph_PARKIN~pUb (left) vs. kinked pUBH in previous PARKIN structures (middle, shown is full-length _Rn_PARKIN, pdb-id 4k95, 16). Superposition indicating IBR repositioning by >20Å (right). (b) Full-length _Rn_PARKIN (top) with core domains under a transparent surface and Ubl as green cartoon, and _Ph_PARKIN~pUb (bottom),with a green circle indicating the putative Ubl binding site; phosphoUb in orange. (c) ITC experiment titrating _Hs_PARKIN Ubl domain into _Hs_PARKINΔUbl without phosphoUb (blue curve, Kd ~39 µM), with phosphoUb present (black curve, Kd not detectable (n.d.)), and _Hs_PARKIN K151E with phosphoUb present (red curve, Kd ~69 µM). Heat release curves (top panel) were shifted in their y-axis position for better visibility. Dissociation constant and curve fitting errors are indicated. All measurements were performed three times with consistent results. (d) Activity of full-length _Hs_PARKIN, _Hs_PARKINΔUbl (137-465), _Hs_PARKINΔUbl2 (80-465) and SUMO-tagged _Hs_PARKINΔUbl2 (80-465) without or with phosphoUb for 1 h (see Fig. 2f, Extended Data Fig. 3f). Experiments were performed three times with consistent results.
Figure 4. PhosphoUb induces PARKIN Ubl phosphorylation and model of PARKIN activation
(a) Detail of the Ubl position on PARKIN. Ubl Ile44 and interacting Leu266 on RING1 are highlighted. The mostly disordered IBR-REP linker hovers atop Ser65 and moves out of the way in _Ph_PARKIN~pUb (compare Fig. 3b). (b) Time course of phosphorylation of _Hs_PARKIN by GST-_Tc_PINK1 (Tribolium castaneum PINK1), in absence and presence of phosphoUb at 37°C, visualised with anti-pSer65 PARKIN antibody (Abcam cat no. ab154995) (top) and Coomassie (below). The experiment was performed three times with consistent results. (c) Experiment as in b but performed at 22°C with lower phosphoUb concentration (5.8 μM). The experiment was performed three times with consistent results. Also see Extended Data Fig. 7c-d.(d) Model of PARKIN activation. PARKIN is autoinhibited (top left) by multiple mechanisms (Extended Data Fig. 1). Inactive PARKIN can interact with phosphoUb, which releases the Ubl domain, destabilises inhibitory interactions and ‘opens’ PARKIN (top row). This process is reversible. PARKIN is phosphorylated by PINK1, either directly (bottom left) or with improved kinetics after phosphoUb binding (bottom middle). Phosphorylated Ubl undergoes a conformational change (Extended Data Fig. 8), probably preventing it from reverting back to the autoinhibited state. Phosphorylation of PARKIN hence stabilises an open, active conformation (bottom right). Thickness of arrows indicates preferred routes. Further information on role of Ubl phosphorylation is in Extended Data Fig. 9 and 10.
Similar articles
- Mechanism of parkin activation by PINK1.
Gladkova C, Maslen SL, Skehel JM, Komander D. Gladkova C, et al. Nature. 2018 Jul;559(7714):410-414. doi: 10.1038/s41586-018-0224-x. Epub 2018 Jun 6. Nature. 2018. PMID: 29995846 Free PMC article. - Binding to serine 65-phosphorylated ubiquitin primes Parkin for optimal PINK1-dependent phosphorylation and activation.
Kazlauskaite A, Martínez-Torres RJ, Wilkie S, Kumar A, Peltier J, Gonzalez A, Johnson C, Zhang J, Hope AG, Peggie M, Trost M, van Aalten DM, Alessi DR, Prescott AR, Knebel A, Walden H, Muqit MM. Kazlauskaite A, et al. EMBO Rep. 2015 Aug;16(8):939-54. doi: 10.15252/embr.201540352. Epub 2015 Jun 25. EMBO Rep. 2015. PMID: 26116755 Free PMC article. - Structure of phosphorylated UBL domain and insights into PINK1-orchestrated parkin activation.
Aguirre JD, Dunkerley KM, Mercier P, Shaw GS. Aguirre JD, et al. Proc Natl Acad Sci U S A. 2017 Jan 10;114(2):298-303. doi: 10.1073/pnas.1613040114. Epub 2016 Dec 22. Proc Natl Acad Sci U S A. 2017. PMID: 28007983 Free PMC article. - Activation of the E3 ubiquitin ligase Parkin.
Caulfield TR, Fiesel FC, Springer W. Caulfield TR, et al. Biochem Soc Trans. 2015 Apr;43(2):269-74. doi: 10.1042/BST20140321. Biochem Soc Trans. 2015. PMID: 25849928 Free PMC article. Review. - PINK1/Parkin-mediated mitophagy in mammalian cells.
Eiyama A, Okamoto K. Eiyama A, et al. Curr Opin Cell Biol. 2015 Apr;33:95-101. doi: 10.1016/j.ceb.2015.01.002. Epub 2015 Feb 17. Curr Opin Cell Biol. 2015. PMID: 25697963 Review.
Cited by
- Autophagy in leukocytes and other cells: mechanisms, subsystem organization, selectivity, and links to innate immunity.
Deretic V. Deretic V. J Leukoc Biol. 2016 Nov;100(5):969-978. doi: 10.1189/jlb.4MR0216-079R. Epub 2016 Aug 4. J Leukoc Biol. 2016. PMID: 27493243 Free PMC article. Review. - Development and characterization of phospho-ubiquitin antibodies to monitor PINK1-PRKN signaling in cells and tissue.
Watzlawik JO, Hou X, Richardson T, Lewicki SL, Siuda J, Wszolek ZK, Cook CN, Petrucelli L, DeTure M, Dickson DW, Antico O, Muqit MMK, Fishman JB, Pirani K, Kumaran R, Polinski NK, Fiesel FC, Springer W. Watzlawik JO, et al. bioRxiv [Preprint]. 2024 Jan 16:2024.01.15.575715. doi: 10.1101/2024.01.15.575715. bioRxiv. 2024. PMID: 38293125 Free PMC article. Updated. Preprint. - The Ubiquitin Proteasome System as a Therapeutic Area in Parkinson's Disease.
Suresh K, Mattern M, Goldberg MS, Butt TR. Suresh K, et al. Neuromolecular Med. 2023 Sep;25(3):313-329. doi: 10.1007/s12017-023-08738-1. Epub 2023 Feb 5. Neuromolecular Med. 2023. PMID: 36739586 Review. - Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment.
Ajoolabady A, Chiong M, Lavandero S, Klionsky DJ, Ren J. Ajoolabady A, et al. Trends Mol Med. 2022 Oct;28(10):836-849. doi: 10.1016/j.molmed.2022.06.007. Epub 2022 Jul 22. Trends Mol Med. 2022. PMID: 35879138 Free PMC article. Review. - The Long and the Short of PTEN in the Regulation of Mitophagy.
Wang L, Lu G, Shen HM. Wang L, et al. Front Cell Dev Biol. 2020 May 13;8:299. doi: 10.3389/fcell.2020.00299. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32478067 Free PMC article. Review.
References
- Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev. 2011;91:1161–1218. - PubMed
- Corti O, Brice A. Mitochondrial quality control turns out to be the principal suspect in parkin and PINK1-related autosomal recessive Parkinson's disease. Curr Opin Neurobiol. 2013;23:100–108. - PubMed
- Koyano F, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials